One-Year Outcomes and Trends over Two Eras of Transcatheter Aortic Valve Implantation in Real-World Practice
Abstract
:1. Introduction
2. Materials and Methods
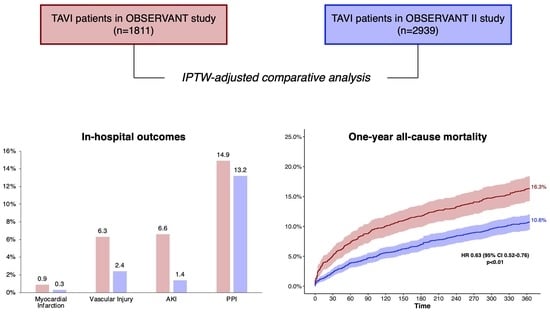
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. OBSERVANT II Investigators
Appendix A.1. Coordination
Appendix A.2. Collaborators for the “Ricerca Finalizzata 2016” (PE-2016-02364619)
Appendix A.3. Representatives of the Scientific Societies
- IFC—Italian Federation of Cardiology
- GISE—Italian Society of Interventional Cardiology
- ITACTA—Italian Association of Cardiothoracic Anesthesia Massimo Baiocchi. Policlinico Sant’Orsola, Bologna, Italy
Appendix A.4. Institutional Collaborations
- National
- Italian Regional Authorities
Appendix A.5. Participating Centers
- A.O.U. Città della Salute e della Scienza di Torino (TO)—Mauro Rinaldi, Stefano Salizzoni.
- A.O. S. Croce e Carle (CN)—Giuseppe Musumeci, Giorgio Baralis.
- A.O. SS. Antonio e Biagio e Cesare Arrigo (AL)—Gianfranco Pistis, Maurizio Reale.
- I.R.C.C.S Policlinico San Donato (San Donato Milanese—MI)—Francesco Bedogni, Giovanni Bianchi.
- I.R.C.C.S Multimedica (Sesto San Giovanni—MI)—Flavio Airoldi, Iassen Michev.
- Fondazione I.R.C.C.S. Policlinico San Matteo (PV)—Maurizio Ferrario, Umberto Canosi.
- ASST Lecco—Ospedale “A. Manzoni” (LC)—Luigi Piatti, Gianluca Tiberti.
- ASST degli Spedali Civili—Presidio Ospedaliero di Brescia (BS)—Federica Ettori (retired), Salvatore Curello, Marianna Adamo.
- I.R.C.C.S Ospedale San Raffaele (MI)—Antonio Colombo, Matteo Montorfano, Marco Ancona.
- ASST Monza & Brianza—Ospedale S. Gerardo (MB)—Virgilio Colombo, Ivan Calchera.
- Fondazione Poliambulanza (BS)—Ornella Leonzi, Diego Maffeo.
- ASST Papa Giovanni XXIII (BG)—Orazio Valsecchi, Federica Roncali, Angelina Vassileva.
- Policlinico di Monza (MB)—Filippo Scalise, Giovanni Sorropago.
- A.O. di Padova—Centro Gallucci (PD)—Giuseppe Tarantini, Alessandro Schiavo.
- Hesperia Hospital (MO)—Giuseppe D’Anniballe, Davide Gabbieri.
- A.O.U. di Parma (PR)—Luigi Vignali, Michela Bollettino.
- A.O.U. Careggi (FI)—Carlo Di Mario, Francesco Meucci.
- A.O.U. Senese—Ospedale Santa Maria alle Scotte (SI)—Carlo Pierli (retired), Massimo Fineschi, Alessandro Iadanza.
- Fondazione Toscana Gabriele Monasterio—Ospedale del Cuore “G. Pasquinucci” (MS)—Sergio Berti, Giuseppa Lo Surdo.
- Ospedale San Filippo Neri (RM)—Giulio Speciale, Andrea Bisciglia.
- Fondazione Policlinico Universitario Agostino Gemelli IRCCS—Università Cattolica del Sacro Cuore (RM)—Carlo Trani, Diana Verdirosi.
- A.O. San Camillo Forlanini (RM)—Roberto Violini, Laura Zappavigna.
- A.O. San Giuseppe Moscati (AV)—Emilio Di Lorenzo, Michele Capasso.
- A.O.U. Federico II (NA)—Giovanni Esposito, Fabio Magliulo.
- A.O.U. OO.RR. San Giovanni di Dio e Ruggi d’Aragona (SA)—Pietro Giudice, Tiziana Attisano.
- A.O.U.C. Policlinico di Bari (BA)—Alessandro Santo Bortone, Emanuela De Cillis.
- A.O.U. Policlinico-Vittorio Emanuele, Università di Catania (CT)—Corrado Tamburino, Marco Barbanti.
- Centro Cuore Morgagni—Pedara (CT)—Sebastiano Immè, Martina Patanè.
References
- Popma, J.J.; Michael Deeb, G.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; Costa, G. Highlights from the 2020 ACC/AHA guidelines on valvular heart disease. EuroIntervention 2021, 16, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; Buccheri, S.; Rodés-Cabau, J.; Gulino, S.; Généreux, P.; Pilato, G.; Dvir, D.; Picci, A.; Costa, G.; Tamburino, C.; et al. Transcatheter aortic valve replacement with new-generation devices: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 245, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Lefevre, T.; Van Belle, E.; Eltchaninoff, H.; Iung, B.; Koning, R.; Motreff, P.; Leprince, P.; Verhoye, J.P.; Manigold, T.; et al. Temporal Trends in Transcatheter Aortic Valve Replacement in France: FRANCE 2 to FRANCE TAVI. J. Am. Coll. Cardiol. 2017, 70, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef] [PubMed]
- Tamburino, C.; Barbanti, M.; D’Errigo, P.; Ranucci, M.; Onorati, F.; Covello, R.D.; Santini, F.; Rosato, S.; Santoro, G.; Fusco, D.; et al. 1-Year Outcomes After Transfemoral Transcatheter or Surgical Aortic Valve Replacement. J. Am. Coll. Cardiol. 2015, 66, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Seccareccia, F.; Tarantini, G.; Bedogni, F.; Berti, S.; Santoro, G.; Tamburino, C.; Ussia, G.P.; Barbanti, M.; Baiocchi, M.; Ranucci, M.; et al. OBSERVANT II: OBservational Study of Effectiveness of transcatheter aortic valve implantation with new geneRation deVices for severe Aortic steNosis Treatment. Study protocol. G. Ital. Cardiol. 2017, 18, 14S–26S. [Google Scholar] [CrossRef]
- Spence, M.S.; Baan, J.; Iacovelli, F.; Martinelli, G.L.; Muir, D.F.; Saia, F.; Bortone, A.S.; Densem, C.G.; Owens, C.G.; van der Kley, F.; et al. Prespecified Risk Criteria Facilitate Adequate Discharge and Long-Term Outcomes After Transfemoral Transcatheter Aortic Valve Implantation. J. Am. Heart Assoc. 2020, 9, e016990. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Barbanti, M.; Picci, A.; Todaro, D.; La Spina, K.; Di Simone, E.; D’Arrigo, P.; Criscione, E.; Valvo, R.; Reddavid, C.; et al. Predictors and Safety of Next-Day Discharge in Unselected Patients Undergoing Transfemoral Transcatheter Aortic Valve Implantation. EuroIntervention 2020, 16, e494–e501. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; Gulino, S.; Costa, G.; Tamburino, C. Optimization and simplification of transcatheter aortic valve implantation therapy. Expert Rev. Cardiovasc. Ther. 2018, 16, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; van Mourik, M.S.; Spence, M.S.; Icovelli, F.; Martinelli, G.L.; Muir, D.F.; Saia, F.; Bortone, A.S.; Densem, C.G.; van der Kley, F.; et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: The multicentre European FAST-TAVI trial. EuroIntervention 2019, 15, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, D.A.; Lauck, S.B.; Cairns, J.A.; Humphries, K.H.; Cook, R.; Welsh, R.; Leipsic, J.; Genereux, P.; Moss, R.; Jue, J.; et al. The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) Clinical Pathway Facilitates Safe Next-Day Discharge Home at Low-, Medium-, and High-Volume Transfemoral Transcatheter Aortic Valve Replacement Centers. JACC Cardiovasc. Interv. 2019, 12, 459–469. [Google Scholar] [CrossRef] [PubMed]






| Before Adjustment | IPTW Adjustment | PSM Adjustment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OBS (n = 1811) | OBS II (n = 2939) | p Value | OBS (n = 1811) | OBS II (n = 2939) | p Value | OBS (n = 1451) | OBS II (n = 1451) | p Value | |
| Age | 83.0 (79.0–86) | 83.0 (79.0–86.0) | 0.892 | 83.0 (78.0–86.0) | 83.0 (79.0–86.0) | 0.429 | 83.0 (79.0–86.0) | 83.0 (79.0–86.0) | 0.941 |
| Female sex | 1064 (58.8) | 1611 (54.8) | <0.01 | 1021 (56.4) | 1652 (56.2) | 0.921 | 836 (57.6) | 824 (56.8) | 0.680 |
| EuroSCORE II | 5.2 (3.3–7.7) | 5.1 (3.1–8.1) | 0.259 | 5.0 (3.0–7.3) | 5.3 (3.2–8.3) | 0.106 | 4.9 (3.1–7.3) | 5.4 (3.2–8.4) | 0.050 |
| BMI | 25.5 (22.9–28.3) | 25.8 (23.2–29.1) | <0.01 | 25.7 (22.9–28.4) | 25.7 (23.2–28.9) | 0.435 | 25.7 (22.9–28.4) | 25.4 (22.9–28.4) | 0.587 |
| GSS 2 or 3 | 436 (24.1) | 634 (21.6) | 0.045 | 393 (21.7) | 655 (22.3) | 0.667 | 321 (22.1) | 313 (21.6) | 0.753 |
| Diabetes | 481 (26.6) | 809 (27.5) | 0.481 | 489 (27.0) | 808 (27.5) | 0.787 | 389 (26.8) | 377 (26.0) | 0.643 |
| Severe renal failure | 220 (12.1) | 303 (10.3) | 0.051 | 187 (10.3) | 309 (10.5) | 0.849 | 158 (10.9) | 155 (10.7) | 0.905 |
| Dialysis | 42 (2.3) | 71 (2.4) | 0.922 | 42 (2.3) | 71 (2.4) | 0.857 | 34 (2.3) | 31 (2.1) | 0.802 |
| COPD | 507 (28.0) | 470 (16.0) | <0.01 | 359 (19.8) | 591 (20.1) | 0.780 | 337 (23.2) | 312 (21.5) | 0.285 |
| Oxygen Therapy | 108 (6.0) | 96 (3.3) | <0.01 | 71 (3.9) | 109 (3.7) | 0.679 | 60 (4.1) | 53 (3.7) | 0.565 |
| Neurological dysf. | 126 (7.0) | 74 (2.5) | <0.01 | 74 (4.1) | 120 (4.1) | 0.997 | 63 (4.3) | 62 (4.3) | 1.000 |
| PAD | 477 (26.3) | 555 (18.9) | <0.01 | 398 (22.0) | 647 (22.0) | 0.970 | 349 (24.1) | 349 (24.1) | 1.000 |
| Liver cirrhosis | 56 (3.1) | 42 (1.4) | <0.01 | 36 (2.0) | 62 (2.1) | 0.811 | 33 (2.3) | 29 (2.0) | 0.700 |
| Active malignancy | 70 (3.9) | 125 (4.3) | 0.547 | 74 (4.1) | 118 (4.0) | 0.929 | 57 (3.9) | 54 (3.7) | 0.847 |
| PH | 319 (17.6) | 151 (5.1) | <0.01 | 181 (10.0) | 270 (9.2) | 0.473 | 136 (9.4) | 129 (8.9) | 0.699 |
| Angina | 49 (2.7) | 138 (4.7) | <0.01 | 78 (4.3) | 118 (4.0) | 0.698 | 44 (3.0) | 50 (3.4) | 0.600 |
| CAD | 554 (30.6) | 751 (25.6) | <0.01 | 505 (27.9) | 802 (27.3) | 0.720 | 420 (28.9) | 414 (28.5) | 0.838 |
| Previous MI | 315 (17.4) | 434 (14.8) | 0.017 | 273 (15.1) | 464 (15.8) | 0.526 | 236 (16.3) | 228 (15.7) | 0.723 |
| Pre. aortic surgery | 93 (5.1) | 107 (3.6) | 0.014 | 78 (4.3) | 120 (4.1) | 0.865 | 67 (4.6) | 75 (5.2) | 0.547 |
| Previous PCI | 482 (26.6) | 416 (14.2) | <0.01 | 330 (18.2) | 558 (19.0) | 0.517 | 322 (22.2) | 312 (21.5) | 0.686 |
| Previous CABG | 228 (12.6) | 299 (10.2) | 0.012 | 194 (10.7) | 320 (10.9) | 0.850 | 168 (11.6) | 177 (12.2) | 0.646 |
| AF | 402 (22.2) | 658 (22.4) | 0.886 | 389 (21.5) | 655 (22.3) | 0.596 | 309 (21.3) | 312 (21.5) | 0.928 |
| RBBB | 111 (6.1) | 201 (6.8) | 0.366 | 129 (7.1) | 200 (6.8) | 0.773 | 98 (6.8) | 95 (6.5) | 0.882 |
| NYHA > 2 | 1205 (66.5) | 2116 (72.0) | <0.01 | 1262 (69.7) | 2054 (69.9) | 0.872 | 985 (67.9) | 988 (68.1) | 0.937 |
| Critical status | 78 (4.3) | 77 (2.6) | <0.01 | 96 (5.3) | 91 (3.1) | 0.128 | 46 (3.2) | 50 (3.4) | 0.756 |
| Hemoglobin (g/dL) | 12.0 (10.0–13.0) | 12.0 (11.0–13.0) | <0.01 | 12.0 (11.0–13.0) | 12.0 (11.0–13.0) | 0.685 | 12.0 (11.0–13.0) | 12.0 (10.0–13.0) | 0.959 |
| Echocardiographic parameters | |||||||||
| LVEF | 55.0 (45.0–60.0) | 55.0 (48.0–60.0) | <0.01 | 55.0 (45.0–60.0) | 55.0 (46.0–60.0) | 0.963 | 55.0 (46.0–60.0) | 55.0 (46.0–60.0) | 0.545 |
| Aortic mean gradient (mmHg) | 48.0 (40.0–58.0) | 46.0 (39.0–55.0) | <0.01 | 47.0 (40.0–56.0) | 47.0 (40.0–55.0) | 0.350 | 48.0 (40.0–57.0) | 47.0 (40.0–56.0) | 0.228 |
| AVA (cm2) | 0.7 (0.5–0.8) | 0.7 (0.5–0.8) | <0.01 | 0.7 (0.5–0.8) | 0.7 (0.5–0.8) | 0.911 | 0.7 (0.5–0.8) | 0.7 (0.5–0.8) | 0.480 |
| Grade 2 + MR | 526 (29.0) | 938 (31.9) | 0.038 | 585 (32.3) | 911 (31.0) | 0.516 | 414 (28.5) | 419 (28.9) | 0.870 |
| OBS | OBS II | HR/SHR (95%CI) | p-Value | |
|---|---|---|---|---|
| IPTW adjustment | N = 1811 | N = 2939 | ||
| All-cause death | 16.3% | 10.6% | 0.63 (0.52–0.76) | <0.001 |
| Rehospitalization for HF | 21.0% | 14.9% | 0.68 (0.58–0.80) | <0.001 |
| Stroke | 3.6% | 3.1% | 0.85 (0.60–1.21) | 0.142 |
| PPI | 18.0% | 16.6% | 0.92 (0.78–1.09) | 0.109 |
| MI | 2.0% | 1.7% | 0.87 (0.55–1.35) | 0.341 |
| PSM adjustment | N = 1451 | N = 1451 | ||
| All-cause death | 16.3% | 11.2% | 0.67 (0.55–0.81) | <0.001 |
| Rehospitalization for HF | 22.5% | 16.1% | 0.69 (0.58–0.81) | <0.001 |
| Stroke | 2.1% | 1.8% | 0.90 (0.62–1.33) | 0.614 |
| PPI | 18.5% | 16.8% | 0.91 (0.77–1.09) | 0.307 |
| MI | 2.1% | 1.8% | 0.84 (0.50–1.41) | 0.501 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, G.; D’Errigo, P.; Rosato, S.; Biancari, F.; Marcellusi, A.; Tarantini, G.; Santoro, G.; Baiocchi, M.; Maffeo, D.; Fiorina, C.; et al. One-Year Outcomes and Trends over Two Eras of Transcatheter Aortic Valve Implantation in Real-World Practice. J. Clin. Med. 2022, 11, 1164. https://doi.org/10.3390/jcm11051164
Costa G, D’Errigo P, Rosato S, Biancari F, Marcellusi A, Tarantini G, Santoro G, Baiocchi M, Maffeo D, Fiorina C, et al. One-Year Outcomes and Trends over Two Eras of Transcatheter Aortic Valve Implantation in Real-World Practice. Journal of Clinical Medicine. 2022; 11(5):1164. https://doi.org/10.3390/jcm11051164
Chicago/Turabian StyleCosta, Giuliano, Paola D’Errigo, Stefano Rosato, Fausto Biancari, Andrea Marcellusi, Giuseppe Tarantini, Gennaro Santoro, Massimo Baiocchi, Diego Maffeo, Claudia Fiorina, and et al. 2022. "One-Year Outcomes and Trends over Two Eras of Transcatheter Aortic Valve Implantation in Real-World Practice" Journal of Clinical Medicine 11, no. 5: 1164. https://doi.org/10.3390/jcm11051164
APA StyleCosta, G., D’Errigo, P., Rosato, S., Biancari, F., Marcellusi, A., Tarantini, G., Santoro, G., Baiocchi, M., Maffeo, D., Fiorina, C., Cerza, F., Baglio, G., Juvonen, T., Badoni, G., Valvo, R., Seccareccia, F., Barbanti, M., Tamburino, C., & on behalf of the OBSERVANT II Research Group. (2022). One-Year Outcomes and Trends over Two Eras of Transcatheter Aortic Valve Implantation in Real-World Practice. Journal of Clinical Medicine, 11(5), 1164. https://doi.org/10.3390/jcm11051164