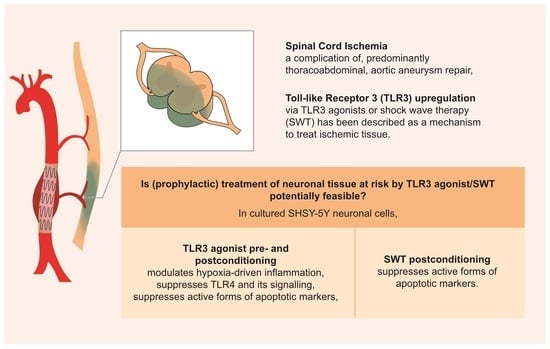
Neuronal Pre- and Postconditioning via Toll-like Receptor 3 Agonist or Extracorporeal Shock Wave Therapy as New Treatment Strategies for Spinal Cord Ischemia: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Oxygen Glucose Deprivation
2.2. Pre- and Postconditioning
2.3. Transfection/Small Interfering RNA (siRNA)
2.4. Quantitative Real-Time PCR (qRT-PCR)
2.5. Western Blot
2.6. Statistical Analysis
3. Results
3.1. Postconditioning of SH-SY5Y Cells Using Poly(I:C) or SWT Causes a Differentiated Genetic Regulation of Hypoxia Inducible Factor 1 Alpha, Proinflammatory Cytokines, TLR3, and Its Downstream Signaling
3.2. Pre- and Postconditioning of poly(I:C) and SWT Lead to Diverging mRNA Levels of Cytokines and HIF1alpha
3.3. Poly(I:C) or SWT in Pre- and Postconditioning Leads to Temporary Suppression of TLR4 and Downstream Signaling
3.4. Ischemic Pre- and Postconditioning via TLR3 Activation Leads to Decreased Apoptotic Markers Early after Reoxygenation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Daye, D.; Walker, T.G. Complications of endovascular aneurysm repair of the thoracic and abdominal aorta: Evaluation and management. Cardiovasc. Diagn. Ther. 2018, 8, S138–S156. [Google Scholar] [CrossRef] [Green Version]
- LeMaire, S.A.; Price, M.D.; Green, S.Y.; Zarda, S.; Coselli, J.S. Results of open thoracoabdominal aortic aneurysm repair. Ann. Cardiothorac. Surg. 2012, 1, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Mohan, V.; Ameta, D.; Gaurav, B.; Haranahalli, P. Aortic aneurysm. J. Transl. Int. Med. 2016, 4, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isselbacher, E.M. Thoracic and abdominal aortic aneurysms. Circulation 2005, 111, 816–828. [Google Scholar] [CrossRef] [Green Version]
- Isselbacher, E.M. The Natural History of Thoracic Aortic Disease: So Much Left to Learn. J. Am. Coll. Cardiol. 2016, 67, 2755–2757. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.S.; Crawford, J.L.; Safi, H.J.; Coselli, J.S.; Hess, K.R.; Brooks, B.; Norton, H.J.; Glaeser, D.H. Thoracoabdominal aortic aneurysms: Preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J. Vasc. Surg. 1986, 3, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Safi, H.J.; Winnerkvist, A.; Miller, C.C., 3rd; Iliopoulos, D.C.; Reardon, M.J.; Espada, R.; Baldwin, J.C. Effect of extended cross-clamp time during thoracoabdominal aortic aneurysm repair. Ann. Thorac. Surg. 1998, 66, 1204–1209. [Google Scholar] [CrossRef]
- Coselli, J.S.; Bozinovski, J.; LeMaire, S.A. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann. Thorac. Surg. 2007, 83, S862–S864; discussion S890–S892. [Google Scholar] [CrossRef]
- Xue, L.; Luo, S.; Ding, H.; Zhu, Y.; Liu, Y.; Huang, W.; Li, J.; Xie, N.; He, P.; Fan, X.; et al. Risk of spinal cord ischemia after thoracic endovascular aortic repair. J. Thorac. Dis. 2018, 10, 6088–6096. [Google Scholar] [CrossRef]
- Heidemann, F.; Tsilimparis, N.; Rohlffs, F.; Debus, E.S.; Larena-Avellaneda, A.; Wipper, S.; Kölbel, T. Staged procedures for prevention of spinal cord ischemia in endovascular aortic surgery. Gefässchirurgie 2018, 23, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Melissano, G.; Civilini, E.; Bertoglio, L.; Calliari, F.; Campos Moraes Amato, A.; Chiesa, R. Angio-CT imaging of the spinal cord vascularisation: A pictorial essay. Eur. J. Vasc. Endovasc. Surg. 2010, 39, 436–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etz, C.D.; Weigang, E.; Hartert, M.; Lonn, L.; Mestres, C.A.; Di Bartolomeo, R.; Bachet, J.E.; Carrel, T.P.; Grabenwöger, M.; Schepens, M.A.; et al. Contemporary spinal cord protection during thoracic and thoracoabdominal aortic surgery and endovascular aortic repair: A position paper of the vascular domain of the European Association for Cardio-Thoracic Surgery. Eur. J. Cardio-Thorac. Surg. 2015, 47, 943–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardone, R.; Pikija, S.; Mutzenbach, J.S.; Seidl, M.; Leis, S.; Trinka, E.; Sellner, J. Current and emerging treatment options for spinal cord ischemia. Drug Discov. Today 2016, 21, 1632–1641. [Google Scholar] [CrossRef]
- Zoli, S.; Roder, F.; Etz, C.D.; Brenner, R.M.; Bodian, C.A.; Lin, H.M.; Di Luozzo, G.; Griepp, R.B. Predicting the risk of paraplegia after thoracic and thoracoabdominal aneurysm repair. Ann. Thorac. Surg. 2010, 90, 1237–1244; discussion 1245. [Google Scholar] [CrossRef]
- Ullery, B.W.; Cheung, A.T.; Fairman, R.M.; Jackson, B.M.; Woo, E.Y.; Bavaria, J.; Pochettino, A.; Wang, G.J. Risk factors, outcomes, and clinical manifestations of spinal cord ischemia following thoracic endovascular aortic repair. J. Vasc. Surg. 2011, 54, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.R.; Coselli, J.S.; Amerman, K.; Bozinovski, J.; Carter, S.A.; Vaughn, W.K.; LeMaire, S.A. Delayed spinal cord deficits after thoracoabdominal aortic aneurysm repair. Ann. Thorac. Surg. 2007, 83, 1345–1355; discussion 1355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Li, J.-x.; Fujino, M.; Zhuang, J.; Li, X.-K. Development and Treatments of Inflammatory Cells and Cytokines in Spinal Cord Ischemia-Reperfusion Injury. Mediat. Inflamm. 2013, 2013, 701970. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.G.; Crawford, E.S.; Hess, K.R.; Coselli, J.S.; Safi, H.J. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J. Vasc. Surg. 1993, 17, 357–368; discussion 368–370. [Google Scholar] [CrossRef]
- Estrera, A.L.; Miller, C.C., 3rd; Chen, E.P.; Meada, R.; Torres, R.H.; Porat, E.E.; Huynh, T.T.; Azizzadeh, A.; Safi, H.J. Descending thoracic aortic aneurysm repair: 12-year experience using distal aortic perfusion and cerebrospinal fluid drainage. Ann. Thorac. Surg. 2005, 80, 1290–1296; discussion 1296. [Google Scholar] [CrossRef]
- Spanos, K.; Kölbel, T.; Kubitz, J.C.; Wipper, S.; Konstantinou, N.; Heidemann, F.; Rohlffs, F.; Debus, S.E.; Tsilimparis, N. Risk of spinal cord ischemia after fenestrated or branched endovascular repair of complex aortic aneurysms. J. Vasc. Surg. 2019, 69, 357–366. [Google Scholar] [CrossRef]
- Anwar, M.A.; Shah, M.; Kim, J.; Choi, S. Recent clinical trends in Toll-like receptor targeting therapeutics. Med. Res. Rev. 2019, 39, 1053–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanza, M.; Campolo, M.; Casili, G.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Sodium Butyrate Exerts Neuroprotective Effects in Spinal Cord Injury. Mol. Neurobiol. 2019, 56, 3937–3947. [Google Scholar] [CrossRef] [PubMed]
- Anttila, J.E.; Whitaker, K.W.; Wires, E.S.; Harvey, B.K.; Airavaara, M. Role of microglia in ischemic focal stroke and recovery: Focus on Toll-like receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Mittermayr, R.; Antonic, V.; Hartinger, J.; Kaufmann, H.; Redl, H.; Téot, L.; Stojadinovic, A.; Schaden, W. Extracorporeal shock wave therapy (ESWT) for wound healing: Technology, mechanisms, and clinical efficacy. Wound Repair Regen. 2012, 20, 456–465. [Google Scholar] [CrossRef]
- Holfeld, J.; Tepeköylü, C.; Reissig, C.; Lobenwein, D.; Scheller, B.; Kirchmair, E.; Kozaryn, R.; Albrecht-Schgoer, K.; Krapf, C.; Zins, K.; et al. Toll-like receptor 3 signalling mediates angiogenic response upon shock wave treatment of ischaemic muscle. Cardiovasc. Res. 2016, 109, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Holfeld, J.; Zimpfer, D.; Albrecht-Schgoer, K.; Stojadinovic, A.; Paulus, P.; Dumfarth, J.; Thomas, A.; Lobenwein, D.; Tepekoylu, C.; Rosenhek, R.; et al. Epicardial shock-wave therapy improves ventricular function in a porcine model of ischaemic heart disease. J. Tissue Eng. Regen. Med. 2014, 10, 1057–1064. [Google Scholar] [CrossRef]
- Lobenwein, D.; Tepeköylü, C.; Kozaryn, R.; Pechriggl, E.J.; Bitsche, M.; Graber, M.; Fritsch, H.; Semsroth, S.; Stefanova, N.; Paulus, P.; et al. Shock Wave Treatment Protects From Neuronal Degeneration via a Toll--Like Receptor 3 Dependent Mechanism: Implications of a First—Ever Causal Treatment for Ischemic Spinal Cord Injury. J. Am. Heart Assoc. 2015, 4, e002440. [Google Scholar] [CrossRef] [Green Version]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef]
- Shi, H.; Gabarin, N.; Hickey, E.; Askalan, R. TLR-3 receptor activation protects the very immature brain from ischemic injury. J. Neuroinflamm. 2013, 10, 874. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.-F.; Fang, H.; Chen, J.; Lin, S.; Liu, Y.; Xiong, X.-Y.; Wang, Y.-C.; Xiong, R.-P.; Lv, F.-L.; Wang, J.; et al. Polyinosinic-Polycytidylic Acid Has Therapeutic Effects against Cerebral Ischemia/Reperfusion Injury through the Downregulation of TLR4 Signaling via TLR3. J. Immunol. 2014, 192, 4783–4794. [Google Scholar] [CrossRef] [Green Version]
- Ryou, M.G.; Mallet, R.T. An In Vitro Oxygen-Glucose Deprivation Model for Studying Ischemia-Reperfusion Injury of Neuronal Cells. Methods Mol. Biol. 2018, 1717, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Gollmann-Tepeköylü, C.; Nägele, F.; Graber, M.; Pölzl, L.; Lobenwein, D.; Hirsch, J.; An, A.; Irschick, R.; Röhrs, B.; Kremser, C.; et al. Shock waves promote spinal cord repair via TLR3. JCI Insight 2020, 5, e134552. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Gollmann-Tepeköylü, C.; Pölzl, L.; Graber, M.; Hirsch, J.; Nägele, F.; Lobenwein, D.; Hess, M.W.; Blumer, M.J.; Kirchmair, E.; Zipperle, J.; et al. miR-19a-3p containing exosomes improve function of ischaemic myocardium upon shock wave therapy. Cardiovasc Res. 2020, 116, 1226–1236. [Google Scholar] [CrossRef]
- Pölzl, L.; Nägele, F.; Graber, M.; Hirsch, J.; Lobenwein, D.; Mitrovic, M.; Mayr, A.; Theurl, M.; Schreinlechner, M.; Pamminger, M.; et al. Safety and efficacy of direct Cardiac Shockwave Therapy in patients with ischemic cardiomyopathy undergoing coronary artery bypass grafting (the CAST-HF trial): Study protocol for a randomized controlled trial. Trials 2020, 21, 447. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobenwein, D.; Huber, R.; Kerbler, L.; Gratl, A.; Wipper, S.; Gollmann-Tepeköylü, C.; Holfeld, J. Neuronal Pre- and Postconditioning via Toll-like Receptor 3 Agonist or Extracorporeal Shock Wave Therapy as New Treatment Strategies for Spinal Cord Ischemia: An In Vitro Study. J. Clin. Med. 2022, 11, 2115. https://doi.org/10.3390/jcm11082115
Lobenwein D, Huber R, Kerbler L, Gratl A, Wipper S, Gollmann-Tepeköylü C, Holfeld J. Neuronal Pre- and Postconditioning via Toll-like Receptor 3 Agonist or Extracorporeal Shock Wave Therapy as New Treatment Strategies for Spinal Cord Ischemia: An In Vitro Study. Journal of Clinical Medicine. 2022; 11(8):2115. https://doi.org/10.3390/jcm11082115
Chicago/Turabian StyleLobenwein, Daniela, Rosalie Huber, Lars Kerbler, Alexandra Gratl, Sabine Wipper, Can Gollmann-Tepeköylü, and Johannes Holfeld. 2022. "Neuronal Pre- and Postconditioning via Toll-like Receptor 3 Agonist or Extracorporeal Shock Wave Therapy as New Treatment Strategies for Spinal Cord Ischemia: An In Vitro Study" Journal of Clinical Medicine 11, no. 8: 2115. https://doi.org/10.3390/jcm11082115
APA StyleLobenwein, D., Huber, R., Kerbler, L., Gratl, A., Wipper, S., Gollmann-Tepeköylü, C., & Holfeld, J. (2022). Neuronal Pre- and Postconditioning via Toll-like Receptor 3 Agonist or Extracorporeal Shock Wave Therapy as New Treatment Strategies for Spinal Cord Ischemia: An In Vitro Study. Journal of Clinical Medicine, 11(8), 2115. https://doi.org/10.3390/jcm11082115