Orthodromic and Antidromic Snare Techniques for Left Ventricular Lead Implantation in Cardiac Resynchronization Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
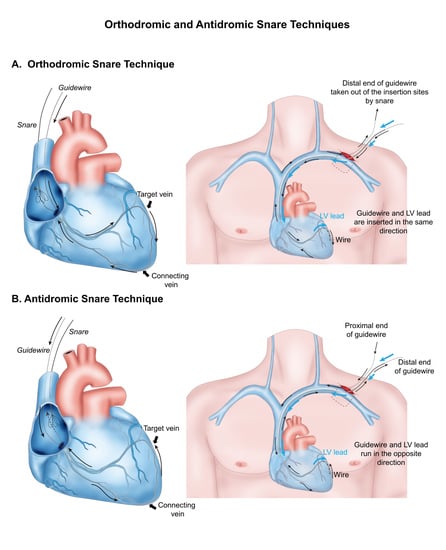
2.2. CRT Implantation and Snare Technique
2.3. Data Collection and Follow-Up
2.4. Study Outcomes and Definitions
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Acute Procedural Outcomes in the Snare Group
3.3. Changes in LV Lead Pacing Threshold and Impedance
3.4. Electrocardiographic and Echocardiographic Responses
3.5. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McAlister, F.A.; Ezekowitz, J.; Hooton, N.; Vandermeer, B.; Spooner, C.; Dryden, D.M.; Page, R.L.; Hlatky, M.A.; Rowe, B.H. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: A systematic review. JAMA 2007, 297, 2502–2514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A., 3rd; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santangeli, P.; Di Biase, L.; Pelargonio, G.; Dello Russo, A.; Casella, M.; Bartoletti, S.; Burkhardt, J.D.; Mohanty, P.; Santarelli, P.; Natale, A. Cardiac resynchronization therapy in patients with mild heart failure: A systematic review and meta-analysis. J. Interv. Card Electrophysiol. 2011, 32, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [Green Version]
- Abraham, W.T.; Gras, D.; Yu, C.M.; Guzzo, L.; Gupta, M.S.; Committee, F.S. Rationale and design of a randomized clinical trial to assess the safety and efficacy of frequent optimization of cardiac resynchronization therapy: The Frequent Optimization Study Using the QuickOpt Method (FREEDOM) trial. Am. Heart J. 2010, 159, 944–948.e941. [Google Scholar] [CrossRef]
- Chung, E.S.; Leon, A.R.; Tavazzi, L.; Sun, J.P.; Nihoyannopoulos, P.; Merlino, J.; Abraham, W.T.; Ghio, S.; Leclercq, C.; Bax, J.J.; et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008, 117, 2608–2616. [Google Scholar] [CrossRef] [Green Version]
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.A.; Cleland, J.; Deharo, J.C.; Delgado, V.; Elliott, P.M.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur. Heart J. 2013, 34, 2281–2329. [Google Scholar]
- Khan, F.Z.; Virdee, M.S.; Palmer, C.R.; Pugh, P.J.; O’Halloran, D.; Elsik, M.; Read, P.A.; Begley, D.; Fynn, S.P.; Dutka, D.P. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: The target study: A randomized, controlled trial. J. Am. Coll. Cardiol. 2012, 59, 1509–1518. [Google Scholar] [CrossRef] [Green Version]
- Saxon, L.A.; Olshansky, B.; Volosin, K.; Steinberg, J.S.; Lee, B.K.; Tomassoni, G.; Guarnieri, T.; Rao, A.; Yong, P.; Galle, E.; et al. Influence of left ventricular lead location on outcomes in the COMPANION study. J. Cardiovasc. Electrophysiol. 2009, 20, 764–768. [Google Scholar] [CrossRef]
- Singh, J.P.; Klein, H.U.; Huang, D.T.; Reek, S.; Kuniss, M.; Quesada, A.; Barsheshet, A.; Cannom, D.; Goldenberg, I.; McNitt, S.; et al. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) trial. Circulation 2011, 123, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Thebault, C.; Donal, E.; Meunier, C.; Gervais, R.; Gerritse, B.; Gold, M.R.; Abraham, W.T.; Linde, C.; Daubert, J.C.; REVERSE Study Group. Sites of left and right ventricular lead implantation and response to cardiac resynchronization therapy observations from the REVERSE trial. Eur. Heart J. 2012, 33, 2662–2671. [Google Scholar] [CrossRef] [Green Version]
- Gras, D.; Bocker, D.; Lunati, M.; Wellens, H.J.; Calvert, M.; Freemantle, N.; Gervais, R.; Kappenberger, L.; Tavazzi, L.; Erdmann, E.; et al. Implantation of cardiac resynchronization therapy systems in the CARE-HF trial: Procedural success rate and safety. Europace 2007, 9, 516–522. [Google Scholar] [CrossRef]
- Morgan, J.M.; Delgado, V. Lead positioning for cardiac resynchronization therapy: Techniques and priorities. Europace 2009, 11 (Suppl. 5), v22–v28. [Google Scholar] [CrossRef]
- Worley, S.J. Challenging Implants Require Tools and Techniques Not Tips and Tricks. Card Electrophysiol. Clin. 2019, 11, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Worley, S.J.; Gohn, D.C.; Pulliam, R.W. Goose neck snare for LV lead placement in difficult venous anatomy. Pacing Clin. Electrophysiol. 2009, 32, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, A.; Menezes, M.; Cortez-Dias, N.; de Sousa, J.; Marques, P. Snare system for left ventricular lead placement in cardiac resynchronization therapy. Rev. Port. Cardiol. 2015, 34, 221–222. [Google Scholar] [CrossRef]
- Nath, R.K.; Raj, A.; Parvatagouda, C.; Pandit, N. Veno-venous loop through coronary sinus for LV lead placement during cardiac resynchronization therapy. Indian Heart J. 2016, 68 (Suppl. 2), S212–S215. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.V.; Deshpande, S.A.; Roul, S.K.; Udyavar, A. Successful use of venovenous snare to fix the wire in a collateral vein for proper placement of the left ventricular lead during cardiac resynchronization therapy: A case report. Eur. Heart J. Case Rep. 2020, 4, 1–7. [Google Scholar] [CrossRef]
- Marques, P.; Nunes-Ferreira, A.; Antonio, P.S.; Aguiar-Ricardo, I.; Lima da Silva, G.; Guimaraes, T.; Bernardes, A.; Santos, I.; Pinto, F.J.; de Sousa, J. Modified snare technique improves left ventricular lead implant success for cardiac resynchronization therapy. J. Cardiovasc. Electrophysiol. 2020, 31, 2954–2963. [Google Scholar] [CrossRef]
- Gwag, H.B.; Park, Y.; Lee, S.S.; Kim, J.S.; Park, K.M.; On, Y.K.; Park, S.J. Efficacy of Cardiac Resynchronization Therapy Using Automated Dynamic Optimization and Left Ventricular-only Pacing. J. Korean Med. Sci. 2019, 34, e187. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Ansalone, G.; Giannantoni, P.; Ricci, R.; Trambaiolo, P.; Fedele, F.; Santini, M. Doppler myocardial imaging to evaluate the effectiveness of pacing sites in patients receiving biventricular pacing. J. Am. Coll. Cardiol. 2002, 39, 489–499. [Google Scholar] [CrossRef]
- Singh, J.P.; Houser, S.; Heist, E.K.; Ruskin, J.N. The coronary venous anatomy: A segmental approach to aid cardiac resynchronization therapy. J. Am. Coll. Cardiol. 2005, 46, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahsan, S.Y.; Saberwal, B.; Lambiase, P.D.; Chaubey, S.; Segal, O.R.; Gopalamurugan, A.B.; McCready, J.; Rogers, D.P.; Lowe, M.D.; Chow, A.W. An 8-year single-centre experience of cardiac resynchronisation therapy: Procedural success, early and late complications, and left ventricular lead performance. Europace 2013, 15, 711–717. [Google Scholar] [CrossRef] [PubMed]






| Total (N = 262) | Snare Group (N = 20) | Conventional Group (N = 242) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, year | 67.4 ± 11.8 | 67.0 ± 12.0 | 67.4 ± 11.8 | 0.883 |
| Male | 168 (64.1) | 10 (50.0) | 158 (65.3) | 0.260 |
| Body mass index, kg/m2 | 23.5 ± 3.6 | 23.0 ± 3.8 | 23.5 ± 3.6 | 0.527 |
| Cardiovascular risk factors | ||||
| Hypertension | 145 (55.3) | 9 (45.0) | 136 (56.2) | 0.463 |
| Diabetes mellitus | 103 (39.3) | 7 (35.0) | 96 (39.7) | 0.863 |
| Chronic kidney disease * | 42 (16.0) | 3 (15.0) | 39 (16.1) | 1.000 |
| Prior myocardial infarction | 37 (14.1) | 2 (10.0) | 35 (14.5) | 0.828 |
| Underlying heart disease | 0.174 | |||
| ICMP | 66 (25.2) | 2 (10.0) | 64 (26.4) | |
| Non-ICMP | 196 (74.8) | 18 (90.0) | 178 (73.6) | |
| ECG findings | ||||
| Atrial fibrillation | 47 (17.9) | 4 (20.0) | 43 (17.8) | 1.000 |
| LBBB | 228 (87.0) | 20 (100.0) | 208 (86.0) | 0.147 |
| Initial QRS duration (msec) | 169 ± 24 | 173 ± 26 | 169 ± 24 | 0.488 |
| Echocardiogram findings | ||||
| Initial LVEF (%) | 28 ± 6 | 30 ± 4 | 27 ± 7 | 0.004 |
| Initial LVESV (mL) | 164 ± 68 | 135 ± 41 | 167 ± 70 | 0.004 |
| Procedure type | 0.960 | |||
| CRT-D | 255 (97.3) | 20 (100.0) | 235 (97.1) | |
| CRT-P | 7 (2.7) | 0 (0.0) | 7 (2.9) | |
| Concurrent medications | ||||
| Beta blocker | 191 (72.9) | 18 (90.0) | 173 (71.5) | 0.126 |
| ACEi, ARB, ARNI | 234 (89.3) | 19 (95.0) | 215 (88.8) | 0.631 |
| MRA | 194 (74.0) | 16 (80.0) | 178 (73.6) | 0.714 |
| Total (N = 262) | Snare Group (N = 20) | Conventional Group (N = 242) | p Value | |
|---|---|---|---|---|
| Follow-up ECG | ||||
| Time to ECG, days | 398 ± 198 | 347 ± 308 | 403 ± 185 | 0.436 |
| Follow-up QRS duration, msec | 136 ± 20 | 134 ± 16 | 136 ± 21 | 0.795 |
| ∆ QRS duration from baseline, msec | −34 ± 26 | −38 ± 19 | −34 ± 27 | 0.446 |
| Follow-up echocardiogram | ||||
| Time to echocardiogram, days | 423 ± 229 | 375 ± 323 | 428 ± 220 | 0.528 |
| LV ejection fraction, % | 40 ± 14 | 42 ± 14 | 39 ± 14 | 0.409 |
| LVEF improvement from baseline, % | 12 ± 13 | 12 ± 13 | 12 ± 13 | 0.929 |
| LVESV, mL | 119 ± 66 | 119 ± 75 | 119 ± 65 | 0.980 |
| LVESV reduction from baseline, % | 27 ± 33 | 18 ± 48 | 28 ± 31 | 0.501 |
| Responder * | 116 (60.7) | 10 (62.5) | 106 (60.6) | 1.000 |
| Super-responder † | 80 (41.9) | 6 (37.5) | 74 (42.3) | 0.915 |
| Snare Group | Conventional Group | Unadjusted HR (95% CI) | Multivariable HR * (95% CI) | p Value | |
|---|---|---|---|---|---|
| Patient number (N = 262) | N = 20 | N = 242 | |||
| Primary outcome † | 25.9% (4) | 30.9% (53) | 0.887 (0.320–2.454) | 0.831 (0.296–2.334) | 0.817 |
| All-cause death | 12.0% (2) | 17.2% (24) | 0.684 (0.161–2.903) | 0.645 (0.148–2.809) | 0.604 |
| Cardiac death | 12.0% (2) | 12.1% (16) | 0.456 (0.104–1.992) | 0.453 (0.100–2.057) | 0.284 |
| Heart failure readmission | 15.6% (2) | 19.3% (34) | 1.181 (0.283–4.923) | 1.107 (0.261–4.686) | 0.819 |
| LVAD implantation | 0% (0) | 4.6% (6) | NA | NA | NA |
| Heart transplantation | 0% (0) | 7.0% (9) | NA | NA | NA |
| LV lead dislodgement or malfunction | 0% (0) | 5.8% (9) | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, S.H.; Kim, H.R.; Chung, T.-W.; Choi, J.-H.; Kim, J.Y.; Park, K.-M.; On, Y.K.; Kim, J.S.; Park, S.-J. Orthodromic and Antidromic Snare Techniques for Left Ventricular Lead Implantation in Cardiac Resynchronization Therapy. J. Clin. Med. 2022, 11, 2133. https://doi.org/10.3390/jcm11082133
Kim J, Lee SH, Kim HR, Chung T-W, Choi J-H, Kim JY, Park K-M, On YK, Kim JS, Park S-J. Orthodromic and Antidromic Snare Techniques for Left Ventricular Lead Implantation in Cardiac Resynchronization Therapy. Journal of Clinical Medicine. 2022; 11(8):2133. https://doi.org/10.3390/jcm11082133
Chicago/Turabian StyleKim, Juwon, Sung Ho Lee, Hye Ree Kim, Tae-Wan Chung, Ji-Hoon Choi, Ju Youn Kim, Kyoung-Min Park, Young Keun On, June Soo Kim, and Seung-Jung Park. 2022. "Orthodromic and Antidromic Snare Techniques for Left Ventricular Lead Implantation in Cardiac Resynchronization Therapy" Journal of Clinical Medicine 11, no. 8: 2133. https://doi.org/10.3390/jcm11082133
APA StyleKim, J., Lee, S. H., Kim, H. R., Chung, T. -W., Choi, J. -H., Kim, J. Y., Park, K. -M., On, Y. K., Kim, J. S., & Park, S. -J. (2022). Orthodromic and Antidromic Snare Techniques for Left Ventricular Lead Implantation in Cardiac Resynchronization Therapy. Journal of Clinical Medicine, 11(8), 2133. https://doi.org/10.3390/jcm11082133