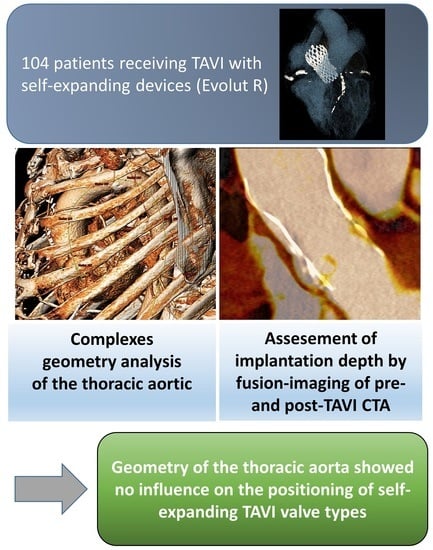
Impact of the Aortic Geometry on TAVI Prosthesis Positioning Using Self-Expanding Valves
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Image Acquisition
2.3. Image Analysis
2.4. Assessment of Aortic Geometry
2.5. Statistical Analysis
3. Results
3.1. Aortic Geometry and Prosthesis Position
3.2. Logistic Regression Analysis
4. Discussion
5. Limitations
6. Conclusions
7. Impact on Daily Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BEV | Balloon-expandable valves |
| CD | Conduction disturbances |
| CTA | Computed tomography angiography |
| ECG | Electrocardiogram |
| LVOT | Left ventricular outflow tract |
| PVL | Paravalvular leakage |
| SEV | Self-expanding valves |
| TAVI | Transcatheter aortic valve implantation |
| THV | Transcatheter heart valve |
References
- Ruile, P.; Pache, G.; Minners, J.; Hein, M.; Neumann, F.-J.; Breitbart, P. Fusion imaging of pre- and post-procedural computed tomography angiography in transcatheter aortic valve implantation patients: Evaluation of prosthesis position and its influence on new conduction disturbances. Eur. Heart J.-Cardiovasc. Imaging 2018, 20, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, P.; Minners, J.; Hein, M.; Schröfel, H.; Neumann, F.-J.; Ruile, P. Implantation depth and its influence on complications after TAVI with self-expanding valves. Int. J. Cardiovasc. Imaging 2021, 37, 3081–3092. [Google Scholar] [CrossRef]
- Petronio, A.S.; Sinning, J.M.; Van Mieghem, N.; Zucchelli, G.; Nickenig, G.; Bekeredjian, R.; De Jaegere, P. Optimal Implantation Depth and Adherence to Guidelines on Permanent Pacing to Improve the Results of Transcatheter Aortic Valve Replacement with the Medtronic CoreValve System: The CoreValve Prospective, International, Post-Market ADVANCE-II Study. Cardiovasc. Interv. 2015, 8, 837–846. [Google Scholar]
- Breitbart, P.; Pache, G.; Minners, J.; Hein, M.; Schröfel, H.; Neumann, F.-J.; Ruile, P. Predictors for low TAVI-prosthesis position assessed by fusion imaging of pre- and post-procedural CT angiography. Clin. Res. Cardiol. 2021, 110, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Gorla, R.; De Marco, F.; Garatti, A.; Bianchi, G.; Rubbio, A.P.; Acerbi, E.; Casenghi, M.; Spagnolo, P.; Brambilla, N.; Testa, L.; et al. Impact of aortic angle on transcatheter aortic valve implantation outcome with Evolut-R, Portico, and Acurate-NEO. Catheter. Cardiovasc. Interv. 2021, 97, 135. [Google Scholar] [CrossRef] [PubMed]
- Rylski, B.; Muñoz, C.; Beyersdorf, F.; Siepe, M.; Reser, D.; Carrel, T.; Schoenhoff, F.; Schlensak, C.; Lescan, M.; Eckstein, H.-H.; et al. How does descending aorta geometry change when it dissects? Eur. J. Cardio-Thoracic Surg. 2017, 53, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Madhwal, S.; Rajagopal, V.; Bhatt, D.L.; Bajzer, C.T.; Whitlow, P.; Kapadia, S.R. Predictors of difficult carotid stenting as determined by aortic arch angiography. J. Invasive Cardiol. 2008, 20, 200–204. [Google Scholar] [PubMed]
- Gode, S.; Akinci, O.; Ustunısık, C.T.; Sen, O.; Kadirogulları, E.; Aksu, T.; Ersoy, B.; Gurbak, I.; Duman, Z.M.; Erentug, V. The role of the angle of the ascending aortic curvature on the development of type A aortic dissection: Ascending aortic angulation and dissection. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 615–620. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.S.; Moon, E.S.; Yoon, C.-S.; Chung, T.-S.; Song, H.-T.; Suh, J.-S.; Lee, Y.H.; Kim, S. Scoliosis Imaging: What Radiologists Should Know. Radiographics 2010, 30, 1823–1842. [Google Scholar] [CrossRef]
- D’ostrevy, N.; Ardellier, F.D.; Cassagnes, L.; Ouchchane, L.; Azarnoush, K.; Camilleri, L.; Sakka, L. The apex of the aortic arch backshifts with aging. Surg. Radiol. Anat. 2017, 39, 703–710. [Google Scholar] [CrossRef]
- Rylski, B.; Desjardins, B.; Moser, W.; Bavaria, J.E.; Milewski, R.K. Gender-related changes in aortic geometry throughout life. Eur. J. Cardio-Thoracic Surg. 2014, 45, 805–811. [Google Scholar] [CrossRef] [Green Version]
- Sherif, M.A.; Abdel-Wahab, M.; Stöcker, B.; Geist, V.; Richardt, D.; Tölg, R.; Richardt, G. Anatomic and Procedural Predictors of Paravalvular Aortic Regurgitation After Implantation of the Medtronic CoreValve Bioprosthesis. J. Am. Coll. Cardiol. 2010, 56, 1623–1629. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.G.; Ferreira, S.M.; Fonseca, P.; Dias, T.; Guerreiro, C.; Barbosa, A.R.; Teixeira, P.; Carvalho, M.; Ferreira, W.; Ferreira, N.D.; et al. Association between implantation depth assessed by computed tomography and new-onset conduction disturbances after transcatheter aortic valve implantation. J. Cardiovasc. Comput. Tomogr. 2017, 11, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Krüger, T.; Boburg, R.S.; Hamdoun, H.; Oikonomou, A.; Bongers, M.N.; Schlensak, C. Development of a multivariable prediction model for patient-adjusted aortic risk morphology. Eur. J. Cardio-Thoracic Surg. 2020, 58, 692–699. [Google Scholar] [CrossRef] [PubMed]





| All Patients (n = 104) | ||
|---|---|---|
| Age | (years) | 82.2 ± 5.2 |
| Female | 69 (66.3) | |
| BMI | (kg/m2) | 27.8 ± 4.9 |
| Logistic Euroscore | (%) | 15.1 ± 11.3 |
| Preexisting conduction disturbances | Pacemaker | 5 (4.8) |
| Total Conduction disturbances | 48 (46.2) | |
| Atrial fibrillation | 29 (27.9) | |
| Aortic valve area | (cm2) | 0.72 ± 0.22 |
| Aortic valve type | Tricuspid | 98 (94.2) |
| Bicuspid | 6 (5.8) | |
| Annulus diameter | (mm) | 23.1 ± 2.3 |
| Grade of calcification of the device landing zone | total | 4.2 ± 1.1 |
| Left coronary cusp | 1.4 ± 0.5 | |
| Right coronary cusp | 1.3 ± 0.5 | |
| Non-coronary cusp | 1.5 ± 0.5 | |
| Ejection fraction pre-interventional | (%) | 50.6 ± 10.3 |
| Access route | Transfemoral | 103 (99.0) |
| Trans-subclavian | 1 (1.0) | |
| Prosthesis size | 23 mm | 5 (4.8) |
| 26 mm | 46 (44.2) | |
| 29 mm | 42 (40.4) | |
| 34 mm | 11 (10.6) | |
| Mean | 4.3 ± 3.0 | |
| Implantation depth below | Left coronary cusp | 4.9 ± 2.8 |
| annulus (mm) | Right coronary cusp | 4.9 ± 3.4 |
| Non-coronary cusp | 3.1 ± 3.5 |
| All Patients (n = 104) | High Position (n = 38) | Low Position (n = 66) | p-Value | ||
|---|---|---|---|---|---|
| Aortic angle | (°) | 49.5 [44.3; 54.8] | 48.5 [42.8; 54.3] | 50.0 [45.0; 55.0] | 0.733 |
| Annular angle | (°) | 111.0 [103.0; 123.0] | 109.5 [103.0; 123.3] | 111.5 [103.0; 120.3] | 0.927 |
| Sinus of Valsalva area | (mm2) | 721.0 [612.3; 816.3] | 645.5 [571.8; 776.3] | 756.5 [658.0; 825.5] | 0.005 |
| Sinus of Valsalva maximum diameter | (mm) | 32.8 ± 3.5 | 31.7 ± 3.4 | 33.4 ± 3.3 | 0.013 |
| Sinus of Valsalva minimum diameter | (mm) | 27.9 ± 3.0 | 26.9 ± 3.0 | 28.5 ± 2.9 | 0.010 |
| Ascending aorta area | (mm2) | 953.1 ± 234.6 | 896.4 ± 254.3 | 985.8 ± 217.8 | 0.061 |
| Ascending aorta mean diameter | (mm) | 34.7 ± 4.1 | 33.6 ± 4.2 | 35.3 ± 3.8 | 0.040 |
| Ascending aorta maximum diameter | (mm) | 35.7 ± 4.1 | 34.5 ± 4.2 | 36.4 ± 3.9 | 0.024 |
| Ascending aorta minimum diameter | (mm) | 33.6 ± 4.0 | 32.7 ± 4.3 | 34.1 ± 3.8 | 0.076 |
| Proximal aortic arch area | (mm2) | 804.6 ± 153.7 | 769.3 ± 143.1 | 825.0 ± 157.0 | 0.075 |
| Proximal aortic arch mean diameter | (mm) | 32.0 ± 3.0 | 31.1 ± 2.8 | 32.5 ± 3.0 | 0.024 |
| Proximal aortic arch maximum diameter | (mm) | 33.4 ± 3.2 | 32.6 ± 2.9 | 33.9 ± 3.3 | 0.035 |
| Proximal aortic arch minimum diameter | (mm) | 30.6 ± 2.9 | 30.0 ± 2.7 | 30.9 ± 3.0 | 0.118 |
| Type of the aortic arch | 0.668 | ||||
| I | n | 15 (14.4) | 4 (10.5) | 11 (16.7) | |
| II | n | 82 (78.8) | 31 (81.6) | 51 (77.3) | |
| III | n | 7 (6.7) | 3 (7.9) | 4 (6.1) | |
| Middle aortic arch area | (mm2) | 591.6 ± 113.3 | 569.5 ± 116.9 | 604.3 ± 110.1 | 0.133 |
| Middle aortic arch mean diameter | (mm) | 27.4 ± 2.5 | 26.9 ± 2.5 | 27.7 ± 2.5 | 0.134 |
| Middle aortic arch maximum diameter | (mm) | 28.9 ± 2.7 | 28.4 ± 2.7 | 29.2 ± 2.7 | 0.159 |
| Middle aortic arch minimum diameter | (mm) | 25.7 ± 2.6 | 25.2 ± 2.7 | 26.0 ± 2.5 | 0.114 |
| Distal aortic arch area | (mm2) | 500.8 ± 93.7 | 481.2 ± 82.0 | 512.1 ± 98.7 | 0.105 |
| Distal aortic arch mean diameter | (mm) | 25.2 ± 2.3 | 24.7 ± 2.2 | 25.4 ± 2.4 | 0.123 |
| Distal aortic arch maximum diameter | (mm) | 26.4 ± 2.5 | 26.0 ± 2.4 | 26.6 ± 2.5 | 0.221 |
| Distal aortic arch minimum diameter | (mm) | 24.0 ± 2.2 | 23.5 ± 1.9 | 24.3 ± 2.3 | 0.090 |
| Descending aorta area | (mm2) | 445.4 ± 94.8 | 425.4 ± 68.7 | 456.9 ± 105.8 | 0.103 |
| Descending aorta area mean diameter | (mm) | 23.8 ± 2.7 | 23.3 ± 1.9 | 24.2 ± 3.1 | 0.131 |
| Descending aorta area maximum diameter | (mm) | 24.9 ± 2.6 | 24.3 ± 1.8 | 25.2 ± 2.9 | 0.087 |
| Descending aorta area minimum diameter | (mm) | 22.7 ± 2.4 | 22.4 ± 2.0 | 22.9 ± 2.6 | 0.296 |
| Length of ascending aorta | (mm) | 91.2 ± 10.3 | 89.3 ± 10.2 | 92.3 ± 10.4 | 0.150 |
| Tortuosity | 0.19 [0.13; 0.24] | 0.20 [0.13; 0.26] | 0.18 [0.13; 0.24] | 0.541 | |
| Aortic distance till middle aortic arch | (mm) | 111.1 ± 12.2 | 108.1 ± 11.0 | 112.8 ± 12.6 | 0.060 |
| Aortic distance till distal aortic arch | (mm) | 126.5 [118.0; 138.0] | 123.5 [116.0; 131.0] | 127.0 [119.0; 139.0] | 0.141 |
| Angle of ascending aortic curvature | (°) | 93.0 [85.3; 103.0] | 92.0 [84.5; 105.0] | 93.0 [85.8; 102.0] | 0.885 |
| Aortic apex arch | (°) | 89.2 ± 10.6 | 87.9 ± 10.1 | 89.9 ± 10.9 | 0.334 |
| Distance from annulus to descending aorta | (mm) | 80.0 [72.0; 90.0] | 76.5 [71.0; 81.8] | 83.0 [72.8; 92.3] | 0.033 |
| Distance from ascending to descending aorta | (mm) | 97.1 ± 12.6 | 93.7 ± 11.9 | 99.0 ± 12.7 | 0.037 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Odds Ratio [95% CI] | p-Value | Odds Ratio [95% CI] | p-Value | |
| Sinus of Valsalva area | 1.004 [1.001–1.007] | 0.015 | 1.002 [0.998–1.006] | 0.335 |
| Ascending aorta maximum diameter | 1.138 [1.014–1.277] | 0.028 | 1.048 [0.870–1.262] | 0.620 |
| Proximal aortic arch mean diameter | 1.187 [1.018–1.384] | 0.028 | 1.041 [0.810–1.338] | 0.754 |
| Distance from annulus to descending aorta | 1.041 [1.002–1.082] | 0.037 | 1.030 [0.973–1.089] | 0.309 |
| Distance from ascending to descending aorta | 1.039 [1.002–1.078] | 0.041 | 0.997 [0.941–1.057] | 0.927 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breitbart, P.; Czerny, M.; Minners, J.; Schröfel, H.; Neumann, F.-J.; Ruile, P. Impact of the Aortic Geometry on TAVI Prosthesis Positioning Using Self-Expanding Valves. J. Clin. Med. 2022, 11, 2259. https://doi.org/10.3390/jcm11082259
Breitbart P, Czerny M, Minners J, Schröfel H, Neumann F-J, Ruile P. Impact of the Aortic Geometry on TAVI Prosthesis Positioning Using Self-Expanding Valves. Journal of Clinical Medicine. 2022; 11(8):2259. https://doi.org/10.3390/jcm11082259
Chicago/Turabian StyleBreitbart, Philipp, Martin Czerny, Jan Minners, Holger Schröfel, Franz-Josef Neumann, and Philipp Ruile. 2022. "Impact of the Aortic Geometry on TAVI Prosthesis Positioning Using Self-Expanding Valves" Journal of Clinical Medicine 11, no. 8: 2259. https://doi.org/10.3390/jcm11082259
APA StyleBreitbart, P., Czerny, M., Minners, J., Schröfel, H., Neumann, F.-J., & Ruile, P. (2022). Impact of the Aortic Geometry on TAVI Prosthesis Positioning Using Self-Expanding Valves. Journal of Clinical Medicine, 11(8), 2259. https://doi.org/10.3390/jcm11082259