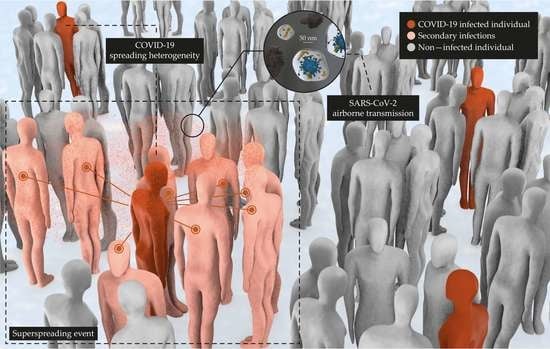
SARS-CoV-2 Droplet and Airborne Transmission Heterogeneity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Included in the Study
2.2. Nasopharyngeal Exudate
2.3. Air Sampling
2.4. Cough Sampling
2.5. Viral RNA Extraction from Masks
2.6. RT-qPCR Analysis
2.6.1. Processing of Environmental Samples
2.6.2. Processing of Swabs Samples
2.6.3. Nucleic Acid Extraction
2.7. Identification of SARS-CoV-2 Variants of Concern by Partial Sequencing of the Spike Gene
2.8. Determination of the Risk of Infection
2.9. Ethical Approval
3. Results and Discussion
3.1. Ct Value Should Not Be Taken as a Predictor of Infectiousness
3.2. Detection of SARS-CoV-2-Laden Bioaerosols
3.3. Do Superspreaders Predominate in the Emission of Bioaerosols with SARS-CoV-2?
3.4. Probable Time Required to Inhale SARS-CoV-2 Bioaerosols to Undergo an Infection
3.5. Viral Spreading Is Heterogeneous
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Boone, S.A.; Gerba, C.P. Significance of Fomites in the Spread of Respiratory and Enteric Viral Disease. Appl. Environ. Microbiol. 2007, 73, 1687–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamadi, M.; Babington-Ashaye, A.; Lefort, A.; Flahault, A. Risks of Infection with Sars-Cov-2 Due to Contaminated Surfaces: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 11019. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.; Holbrook, M.; Gamble, A.; Williamson, B.; Munster, V. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Hirose, R.; Itoh, Y.; Ikegaya, H.; Miyazaki, H.; Watanabe, N.; Yoshida, T.; Bandou, R.; Daidoji, T.; Nakaya, T. Differences in Environmental Stability among SARS-CoV-2 Variants of Concern: Omicron Has Higher Stability. bioRxiv 2022, arXiv:476607. [Google Scholar] [CrossRef]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L.; the Healthcare Infection Control Practices Advisory Committee. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings (Updated July 2019); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019; pp. 1–232.
- Wells, W.F. Airborne Contagion and Air Hygiene: An Ecological Study of Droplet Infections. JAMA 1955, 159, 90. [Google Scholar] [CrossRef]
- Hinds, W. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Yeh, H.; Liu, B. Aerosol Filtraton by Fibrous Filters. I: Theoretical. J. Aerosol Sci. 1974, 5, 191–204. [Google Scholar] [CrossRef]
- Yeh, H.; Liu, B. Aerosol Filtraton by Fibrous Filters. II: Experimental. J. Aerosol Sci. 1974, 5, 205–217. [Google Scholar] [CrossRef]
- Johnson, G.R.; Morawska, L. The Mechanism of Breath Aerosol Formation. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Johnson, G.R.; Morawska, L.; Ristovski, Z.D.; Hargreaves, M.; Mengersen, K.; Chao, C.Y.H.; Wan, M.P.; Li, Y.; Xie, X.; Katoshevski, D.; et al. Modality of Human Expired Aerosol Size Distributions. J. Aerosol Sci. 2011, 42, 839–851. [Google Scholar] [CrossRef]
- Asadi, S.; Wexler, A.S.; Cappa, C.D.; Barreda, S.; Bouvier, N.M.; Ristenpart, W.D. Aerosol Emission and Superemission during Human Speech Increase with Voice Loudness. Sci. Rep. 2019, 9, 2348. [Google Scholar] [CrossRef] [Green Version]
- Dommer, A.; Casalino, L.; Kearns, F.; Rosenfeld, M.; Wauer, N.; Ahn, S.-H.; Russo, J.; Oliveira, S.; Morris, C.; Bogetti, A.; et al. #COVIDisAirborne: AI-Enabled Multiscale Computational Microscopy of Delta SARS-CoV-2 in a Respiratory Aerosol. bioRxiv 2021, arXiv:468428. [Google Scholar] [CrossRef]
- Nicas, M.; Nazaroff, W.W.; Hubbard, A. Toward Understanding the Risk of Secondary Airborne Infection: Emission of Respirable Pathogens. J. Occup. Environ. Hyg. 2005, 2, 143–154. [Google Scholar] [CrossRef]
- Laporte, M.; Raeymaekers, V.; van Berwaer, R.; Vandeput, J.; Marchand-Casas, I.; Thibaut, H.J.; van Looveren, D.; Martens, K.; Hoffmann, M.; Maes, P.; et al. The SARS-CoV-2 and Other Human Coronavirus Spike Proteins Are Fine-Tuned towards Temperature and Proteases of the Human Airways. PLoS Pathog. 2021, 17, e1009500. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Jimenez, J.L.; Prather, K.A.; Tufekci, Z.; Fisman, D.; Schooley, R. Ten Scientific Reasons in Support of Airborne Transmission of SARS-CoV-2. Lancet 2021, 397, 1603–1605. [Google Scholar] [CrossRef]
- Kutter, J.S.; de Meulder, D.; Bestebroer, T.M.; Lexmond, P.; Mulders, A.; Richard, M.; Fouchier, R.A.M.; Herfst, S. SARS-CoV and SARS-CoV-2 Are Transmitted through the Air between Ferrets over More than One Meter Distance. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Sia, S.F.; Yan, L.M.; Chin, A.W.H.; Fung, K.; Choy, K.T.; Wong, A.Y.L.; Kaewpreedee, P.; Perera, R.A.P.M.; Poon, L.L.M.; Nicholls, J.M.; et al. Pathogenesis and Transmission of SARS-CoV-2 in Golden Hamsters. Nature 2020, 583, 834–838. [Google Scholar] [CrossRef]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of Ferrets, Cats, Dogs, and Other Domesticated Animals to SARS-Coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef] [Green Version]
- Lewis, B.D. The Superspreading Problem. Nature 2021, 950, 544–548. [Google Scholar] [CrossRef]
- Eichler, N.; Thornley, C.; Swadi, T.; Devine, T.; McElnay, C.; Sherwood, J.; Brunton, C.; Williamson, F.; Freeman, J.; Berger, S.; et al. Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 during Border Quarantine and Air Travel, New Zealand (Aotearoa). Emerg. Infect. Dis. 2021, 27, 1274–1278. [Google Scholar] [CrossRef]
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 Transmission from People without COVID-19 Symptoms. JAMA Netw. Open 2021, 4, e2035057. [Google Scholar] [CrossRef]
- Bulfone, T.C.; Malekinejad, M.; Rutherford, G.W.; Razani, N. Outdoor Transmission of SARS-CoV-2 and Other Respiratory Viruses: A Systematic Review. J. Infect. Dis. 2021, 223, 550–561. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions (accessed on 21 April 2022).
- Kim, J.-M.; Chung, Y.-S.; Jo, H.J.; Lee, N.-J.; Kim, M.S.; Woo, S.H.; Park, S.; Kim, J.W.; Kim, H.M.; Han, M.-G. Article History: Identification of Coronavirus Isolated from a Patient in Korea with COVID-19 Osong Public Health and Research Perspectives. Public Health Res. Perspect 2020, 11, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.B.; Kwon, N.J.; Choi, S.J.; Kang, C.K.; Choe, P.G.; Kim, J.Y.; Yun, J.; Lee, G.W.; Seong, M.W.; Kim, N.J.; et al. Virus Isolation from the First Patient with SARS-CoV-2 in Korea. J. Korean Med. Sci. 2020, 35, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological Assessment of Hospitalized Patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.U. Minimum Sizes of Respiratory Particles Carrying SARS-CoV-2 and the Possibility of Aerosol Generation. Int. J. Environ. Res. Public Health 2020, 17, 6960. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic Analysis of SARS-CoV-2 in Two Wuhan Hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef]
- Shinya, K.; Ebina, M.; Yamada, S.; Ono, M.; Kasai, N.; Kawaoka, Y. Influenza Virus Receptors in the Human Airway. Nature 2006, 440, 435–436. [Google Scholar] [CrossRef]
- Chao, C.Y.H.; Wan, M.P.; Morawska, L.; Johnson, G.R.; Ristovski, Z.D.; Hargreaves, M.; Mengersen, K.; Corbett, S.; Li, Y.; Xie, X.; et al. Characterization of Expiration Air Jets and Droplet Size Distributions Immediately at the Mouth Opening. J. Aerosol Sci. 2009, 40, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Stadnytskyi, V.; Bax, C.E.; Bax, A.; Anfinrud, P. The Airborne Lifetime of Small Speech Droplets and Their Potential Importance in SARS-CoV-2 Transmission. Proc. Natl. Acad. Sci. USA 2020, 117, 3–5. [Google Scholar] [CrossRef]
- Shao, S.; Zhou, D.; He, R.; Li, J.; Zou, S.; Mallery, K.; Kumar, S.; Yang, S.; Hong, J. Risk Assessment of Airborne Transmission of COVID-19 by Asymptomatic Individuals under Different Practical Settings. J. Aerosol Sci. 2021, 151, 105661. [Google Scholar] [CrossRef]
- Fabian, P.; Brain, J.; Houseman, E.; Gern, J.; Milton, D. Origin of Exhaled Breath Particles from Healthy and Human Rhinovirus-Infected Subjects. J. Aerosol. Med. Pulm. Drug Deliv. 2011, 24, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne Transmission of Respiratory Viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.A.; Koutrakis, P.; Martins, M.A.G.; Lemos, B.; Dowd, S.E.; Sunderland, E.M.; Garshick, E. Characterization of Hospital Airborne SARS-CoV-2. Respir. Res. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Ding, Z.; Qian, H.; Xu, B.; Huang, Y.; Miao, T.; Yen, H.L.; Xiao, S.; Cui, L.; Wu, X.; Shao, W.; et al. Toilets Dominate Environmental Detection of Severe Acute Respiratory Syndrome Coronavirus 2 in a Hospital. Sci. Total Environ. 2021, 753, 141710. [Google Scholar] [CrossRef]
- Lednicky, J.; Shankar, S.; Elbadry, M.; Gibson, J.; Alam, M.; Stephenson, C.; Eiguren, A.; Glenn, J.; Mavian, C.; Salemi, M.; et al. Collection of SARS-CoV-2 Virus from the Air of a Clinic Within a University Student Health Care Center and Analyses of the Viral Genomic Sequence. Aerosol Air Qual. Res. 2020, 20, 1167–1171. [Google Scholar] [CrossRef]
- Chia, P.Y.; Coleman, K.K.; Tan, Y.K.; Ong, S.W.X.; Gum, M.; Lau, S.K.; Lim, X.F.; Lim, A.S.; Sutjipto, S.; Lee, P.H.; et al. Detection of Air and Surface Contamination by SARS-CoV-2 in Hospital Rooms of Infected Patients. Nat. Commun. 2020, 11, 2800. [Google Scholar] [CrossRef]
- Chen, G.M.; Ji, J.J.; Jiang, S.; Xiao, Y.Q.; Zhang, R.L.; Huang, D.N.; Liu, H.; Yu, S.Y. Detecting Environmental Contamination of Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Isolation Wards and Fever Clinics. Biomed. Environ. Sci. 2020, 33, 943–947. [Google Scholar] [CrossRef]
- Santarpia, J.L.; Rivera, D.N.; Herrera, V.L.; Morwitzer, M.J.; Creager, H.M.; Santarpia, G.W.; Crown, K.K.; Brett-Major, D.M.; Schnaubelt, E.R.; Broadhurst, M.J.; et al. Aerosol and Surface Contamination of SARS-CoV-2 Observed in Quarantine and Isolation Care. Sci. Rep. 2020, 10, 12732. [Google Scholar] [CrossRef]
- Zhou, A.J.; Otter, J.A.; Price, J.R.; Cimpeanu, C.; Garcia, M.; Kinross, J.; Boshier, P.R.; Mason, S.; Bolt, F.; Alison, H.; et al. Investigating SARS-CoV-2 Surface and Air Contamination in an Acute Healthcare 2 Setting during the Peak of the COVID-19 Pandemic in London. medRxiv 2020, arXiv:20110346, 1–24. [Google Scholar] [CrossRef]
- Lei, H.; Ye, F.; Liu, X.; Huang, Z.; Ling, S.; Jiang, Z.; Cheng, J.; Huang, X.; Wu, Q.; Wu, S.; et al. SARS-CoV-2 Environmental Contamination Associated with Persistently Infected COVID-19 Patients. Influenza Other Respir. Viruses 2020, 14, 688–699. [Google Scholar] [CrossRef]
- Moore, G.; Rickard, H.; Stevenson, D.; Aranega-Bou, P.; Pitman, J.; Crook, A.; Davies, K.; Spencer, A.; Burton, C.; Easterbrook, L.; et al. Detection of SARS-CoV-2 within the Healthcare Environment: A Multi-Centre Study Conducted during the First Wave of the COVID-19 Outbreak in England. J. Hosp. Infect. 2021, 108, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.; Gonçalves, J.; Isabel, A.; Lopes, B.; Esteves, N.A.; Emanuel, G.; Bamba, E.; Maria, S.; Branco, P.T.B.S.; Soares, R.R.G.; et al. Evidence of Air and Surface Contamination with SARS-CoV-2 in a Major Hospital in Portugal. Int. J. Environ. Res. Public Health 2022, 19, 525. [Google Scholar]
- Department of Infectious Disease, Imperial College London, London, UK. Evaluation of SARS-CoV-2 Air Contamination in Hospitals with Coriolis μ Air Sampler. 2020. Available online: https://www.bertin-instruments.com/air-samplers/application-center/evaluation-of-sars-cov-2-air-contamination-in-hospitals-with-coriolis-micro-air-sampler/ (accessed on 5 February 2022).
- Mallach, G.; Kasloff, S.B.; Kovesi, T.; Kumar, A.; Kulka, R.; Krishnan, J.; Robert, B.; McGuinty, M.; den Otter-Moore, S.; Yazji, B.; et al. Aerosol SARS-CoV-2 in Hospitals and Long-Term Care Homes during the COVID-19 Pandemic. PLoS ONE 2021, 16, e0258151. [Google Scholar] [CrossRef] [PubMed]
- Winslow, R.L.; Zhou, J.; Windle, E.F.; Nur, I.; Lall, R.; Ji, C.; Millar, J.E.; Dark, P.; Naisbitt, J.; Simonds, A.; et al. SARS-CoV-2 Environmental Contamination from Hospitalised COVID-19 Patients Receiving Aerosol Generating Procedures. Thorax 2022, 77, 259–267. [Google Scholar] [CrossRef]
- Kenarkoohi, A.; Noorimotlagh, Z.; Falahi, S.; Amarloei, A.; Abbas, S. Hospital Indoor Air Quality Monitoring for the Detection of SARS-CoV-2 (COVID-19) Virus. Sci. Total Environ. 2020, 748, 141324. [Google Scholar] [CrossRef]
- Ma, J.; Qi, X.; Chen, H.; Li, X.; Zhang, Z.; Wang, H.; Sun, L.; Zhang, L.; Guo, J.; Morawska, L.; et al. Exhaled Breath Is a Significant Source of SARS-CoV-2 Emission. medRxiv 2020, 1–8. [Google Scholar] [CrossRef]
- Styczynski, A.; Hemlock, C.; Hoque, K.I.; Verma, R.; LeBoa, C.; Bhuiyan, M.O.F.; Nag, A.; Harun, M.G.D.; Amin, M.B.; Andrews, J.R. Ventilation and Detection of Airborne SARS-CoV-2: Elucidating High-Risk Spaces in Naturally Ventilated Healthcare Settings. medRxiv 2021. [Google Scholar] [CrossRef]
- Dumont-Leblond, N.; Veillette, M.; Mubareka, S.; Yip, L.; Longtin, Y.; Jouvet, P.; Paquet Bolduc, B.; Godbout, S.; Kobinger, G.; McGeer, A.; et al. Low Incidence of Airborne SARS-CoV-2 in Acute Care Hospital Rooms with Optimized Ventilation. Emerg. Microbes Infect. 2020, 9, 2597–2605. [Google Scholar] [CrossRef]
- Song, Z.G.; Chen, Y.M.; Wu, F.; Xu, L.; Wang, B.F.; Shi, L.; Chen, X.; Dai, F.H.; She, J.L.; Chen, J.M.; et al. Identifying the Risk of SARS-CoV-2 Infection and Environmental Monitoring in Airborne Infectious Isolation Rooms (AIIRs). Virol. Sin. 2020, 35, 785–792. [Google Scholar] [CrossRef]
- Nissen, K.; Krambrich, J.; Akaberi, D.; Hoffman, T.; Ling, J.; Lundkvist, Å.; Svensson, L.; Salaneck, E. Long-Distance Airborne Dispersal of SARS-CoV-2 in COVID-19 Wards. Sci. Rep. 2020, 10, 19589. [Google Scholar] [CrossRef]
- Moreno, T.; Pintó, R.M.; Bosch, A.; Moreno, N.; Alastuey, A.; Minguillón, M.C.; Anfruns-Estrada, E.; Guix, S.; Fuentes, C.; Buonanno, G.; et al. Tracing Surface and Airborne SARS-CoV-2 RNA inside Public Buses and Subway Trains. Environ. Int. 2021, 147, 106326. [Google Scholar] [CrossRef]
- Barbieri, P.; Zupin, L.; Licen, S.; Torboli, V.; Semeraro, S.; Cozzutto, S.; Palmisani, J.; Di Gilio, A.; de Gennaro, G.; Fontana, F.; et al. Molecular Detection of SARS-CoV-2 from Indoor Air Samples in Environmental Monitoring Needs Adequate Temporal Coverage and Infectivity Assessment. Environ. Res. 2021, 198, 111200. [Google Scholar] [CrossRef]
- Ryan, D.J.; Toomey, S.; Madden, S.F.; Casey, M.; Breathnach, O.S.; Morris, P.G.; Grogan, L.; Branagan, P.; Costello, R.W.; De Barra, E.; et al. Use of exhaled breath condensate (EBC) in the diagnosis of SARS-COV-2 (COVID-19). Thorax 2021, 76, 86–88. [Google Scholar] [CrossRef]
- Hu, J.; Lei, C.; Chen, Z.; Liu, W.; Hu, X.; Pei, R.; Su, Z.; Deng, F.; Huang, Y.; Sun, X.; et al. Distribution of Airborne SARS-CoV-2 and Possible Aerosol Transmission in Wuhan Hospitals, China. Natl. Sci. Rev. 2020, 7, 1865–1867. [Google Scholar] [CrossRef]
- Faridi, S.; Niazi, S.; Sadeghi, K.; Naddafi, K.; Yavarian, J.; Shamsipour, M.; Jandaghi, N.Z.S.; Sadeghniiat, K.; Nabizadeh, R.; Yunesian, M.; et al. A Field Indoor Air Measurement of SARS-CoV-2 in the Patient Rooms of the Largest Hospital in Iran. Sci. Total Environ. 2020, 725, 138401. [Google Scholar] [CrossRef]
- Cheng, V.; Wong, S.; Chan, V.; Chun, S.; Chen, J.; Yip, C.; Chan, K.; Chu, H.; Chung, T.; Sridhar, S.; et al. Air and Environmental Sampling for SARS-CoV-2 around Hospitalized Patients with Coronavirus Disease 2019 (COVID-19). Infect. Control Hosp. Epidemiol. 2020, 41, 1258–1265. [Google Scholar] [CrossRef]
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.T.; Wong, M.S.Y.; Marimuthu, K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) from a Symptomatic Patient. JAMA J. Am. Med. Assoc. 2020, 323, 1610–1612. [Google Scholar] [CrossRef] [Green Version]
- Döhla, M.; Wilbring, G.; Schulte, B.; Kümmerer, B.; Diegmann, C.; Sib, E.; Richter, E.; Haag, A.; Engelhart, S.; Eis-Hübinger, A.; et al. SARS-CoV-2 in Environmental Samples of Quarantined Households. medRxiv 2020, 49, 1–19. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Jin, X.; Tian, J.; Liu, J.; Mao, Y. Environmental Contamination by SARS-CoV-2 in a Designated Hospital for Coronavirus Disease 2019. Am. J. Infect. Control 2020, 48, 910–914. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Jiang, L.; Wang, H. Aerosol and Environmental Surface Monitoring for SARS-CoV-2 RNA in a Designated Hospital for Severe COVID-19 Patients. Epidemiol. Infect. 2020, 148, E154. [Google Scholar] [CrossRef]
- Ahn, J.Y.; An, S.; Sohn, Y.; Cho, Y.; Hyun, J.H.; Baek, Y.J.; Kim, M.H.; Jeong, S.J.; Kim, J.H.; Ku, N.S.; et al. Environmental Contamination in the Isolation Rooms of COVID-19 Patients with Severe Pneumonia Requiring Mechanical Ventilation or High-Flow Oxygen Therapy. J. Hosp. Infect. 2020, 106, 570–576. [Google Scholar] [CrossRef]
- Lane, M.A.; Brownsword, E.A.; Morgan, J.S.; Babiker, A.; Vanairsdale, S.A.; Lyon, G.M.; Mehta, A.K.; Ingersoll, J.M.; Lindsley, W.G.; Kraft, C.S. Bioaerosol Sampling of a Ventilated Patient with COVID-19. Am. J. Infect. Control 2020, 48, 1540–1542. [Google Scholar] [CrossRef]
- Yang, W.; Marr, L.C. Dynamics of Airborne Influenza A Viruses Indoors and Dependence on Humidity. PLoS ONE 2011, 6, e21481. [Google Scholar] [CrossRef] [Green Version]
- Tellier, R.; Li, Y.; Cowling, B.J.; Tang, J.W. Recognition of Aerosol Transmission of Infectious Agents: A Commentary. BMC Infect. Dis. 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Vejerano, E.P.; Marr, L.C. Physico-Chemical Characteristics of Evaporating Respiratory Fluid Droplets. J. R. Soc. Interface 2018, 15, 20170939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Marr, L.C. Mechanisms by Which Ambient Humidity May Affect Viruses in Aerosols. Appl. Environ. Microbiol. 2012, 78, 6781–6788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orr, C.; Hurd, F.K.; Corbett, W.J. Aerosol size and relative humidity. J. Colloid Sci. 1958, 13, 472–482. [Google Scholar] [CrossRef]
- Marr, L.C.; Tang, J.W.; Van Mullekom, J.; Lakdawala, S.S. Mechanistic Insights into the Effect of Humidity on Airborne Influenza Virus Survival, Transmission and Incidence. J. R. Soc. Interface 2019, 16, 20180298. [Google Scholar] [CrossRef]
- Ahlawat, A.; Wiedensohler, A.; Mishra, S.K. An Overview on the Role of Relative Humidity in Airborne Transmission of Sars-Cov-2 in Indoor Environments. Aerosol Air Qual. Res. 2020, 20, 1856–1861. [Google Scholar] [CrossRef]
- Fears, A.C.; Klimstra, W.B.; Duprex, P.; Hartman, A.; Weaver, S.C.; Plante, K.S.; Mirchandani, D.; Plante, J.A.; Aguilar, P.V.; Fernández, D.; et al. Persistence of Severe Acute Respiratory Syndrome Coronavirus 2 in Aerosol Suspensions. Emerg. Infect. Dis. 2020, 26, 2168–2171. [Google Scholar] [CrossRef]
- Smither, S.; Eastaugh, L.; Findlay, J.; Lever, M. Experimental Aerosol Survival of SARS-CoV-2 in Artificial Saliva and Tissue Culture Media at Medium and High Humidity. Emerg. Microbes Infect. 2020, 9, 1415–1417. [Google Scholar] [CrossRef]
- Schuit, M.; Ratnesar-Shumate, S.; Yolitz, J.; Williams, G.; Weaver, W.; Green, B.; Miller, D.; Krause, M.; Beck, K.; Wood, S.; et al. Airborne SARS-CoV-2 Is Rapidly Inactivated by Simulated Sunlight. J. Infect. Dis. 2020, 222, 564–571. [Google Scholar] [CrossRef]
- Schuit, M.; Gardner, S.; Wood, S.; Bower, K.; Williams, G.; Freeburger, D.; Dabisch, P. The Influence of Simulated Sunlight on the Inactivation of Influenza Virus in Aerosols. J. Infect. Dis. 2021, 221, 372–378. [Google Scholar] [CrossRef]
- Zhao, Y.; Aarnink, A.J.A.; Dijkman, R.; Fabri, T.; de Jong, M.C.M.; Groot Koerkamp, P.W.G. Effects of Temperature, Relative Humidity, Absolute Humidity, and Evaporation Potential on Survival of Airborne Gumboro Vaccine Virus. Appl. Environ. Microbiol. 2012, 78, 1048–1054. [Google Scholar] [CrossRef] [Green Version]
- Oswin, H.P.; Haddrell, A.E.; Otero-fernandez, M.; Mann, J.F.S.; Cogan, T.A.; Hilditch, T.; Tian, J.; Hardy, D.; Hill, D.J.; Finn, A.; et al. The Dynamics of SARS-CoV-2 Infectivity with Changes in Aerosol Microenvironment. medRxiv 2022, arXiv:22268944. [Google Scholar] [CrossRef]
- Benito, A.A.; Lázaro, S.; Arnal, J.L. ¿Circula El SARS-CoV-2 En La Ganadería Nacional? Albéitar 2021, 242, 8–10. [Google Scholar]
- Corman, V.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Luisa Schmidt, M.; et al. Detection of 2019-NCoV by RT-PCR. Eurosurveillance 2020, 25, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Riley, E.; Murphy, G.; Riley, R. Airborne Spread of Measles in a Suburban Elementary School. Am. J. Epidemiol. 1978, 107, 421–432. [Google Scholar] [CrossRef]
- Peng, Z.; Jimenez, J.L. Exhaled CO2as a COVID-19 Infection Risk Proxy for Different Indoor Environments and Activities. Environ. Sci. Technol. Lett. 2021, 8, 392–397. [Google Scholar] [CrossRef]
- Cortellessa, G.; Stabile, L.; Arpino, F.; Faleiros, D.E.; van den Bos, W.; Morawska, L.; Buonanno, G. Close Proximity Risk Assessment for SARS-CoV-2 Infection. Sci. Total Environ. 2021, 794, 148749. [Google Scholar] [CrossRef]
- Gale, P. Thermodynamic Equilibrium Dose–response Models for MERS-CoV Infection Reveal a Potential Protective Role of Human Lung Mucus but Not for SARS-CoV-2. Microb. Risk Anal. 2020, 16, 100140. [Google Scholar] [CrossRef]
- Gupta, N.; Augustine, S.; Narayan, T.; O’Riordan, A.; Das, A.; Kumar, D.; Luong, J.H.T.; Malhotra, B.D. Point-of-Care PCR Assays for COVID-19 Detection. Biosensors 2021, 11, 141. [Google Scholar] [CrossRef]
- Arnaout, R.; Lee, R.A.; Lee, G.R.; Callahan, C.; Yen, C.F.; Smith, K.P.; Arora, R.; Kirby, J.E. SARS-CoV2 Testing: The Limit of Detection Matters. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA J. Am. Med. Assoc. 2020, 323, 1843–1844. [Google Scholar] [CrossRef] [Green Version]
- Kucirka, L.M.; Lauer, S.A.; Laeyendecker, O.; Boon, D.; Lessler, J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure. Ann. Intern. Med. 2020, 173, 262–267. [Google Scholar] [CrossRef]
- Pujadas, E.; Chaudhry, F.; McBride, R.; Richter, F.; Zhao, S.; Wajnberg, A.; Nadkarni, G.; Glicksberg, B.S.; Houldsworth, J.; Cordon-Cardo, C. SARS-CoV-2 Viral Load Predicts COVID-19 Mortality. Lancet Respir. Med. 2020, 8, e70. [Google Scholar] [CrossRef]
- Rao, S.N.; Manissero, D.; Steele, V.R.; Pareja, J. A Narrative Systematic Review of the Clinical Utility of Cycle Threshold Values in the Context of COVID-19. Infect. Dis. Ther. 2020, 9, 573–586. [Google Scholar] [CrossRef]
- Trunfio, M.; Venuti, F.; Alladio, F.; Longo, B.M.; Burdino, E.; Cerutti, F.; Ghisetti, V.; Bertucci, R.; Picco, C.; Bonora, S.; et al. Diagnostic SARS-CoV-2 Cycle Threshold Value Predicts Disease Severity, Survival, and Six-Month Sequelae in COVID-19 Symptomatic Patients. Diagnostics 2021, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Magleby, R.; Westblade, L.F.; Trzebucki, A.; Simon, M.S.; Rajan, M.; Park, J.; Goyal, P.; Safford, M.M.; Satlin, M.J. Impact of Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load on Risk of Intubation and Mortality Among Hospitalized Patients With Coronavirus Disease 2019. Clin. Infect. Dis. 2021, 73, e4197–e4205. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, A.M.; Obeid, D.A.; Alaifan, T.A.; Taha, M.T.; Alhothali, M.T.; Alzahrani, F.A.; Albarrag, A.M. Assessment of 12 Qualitative RT-PCR Commercial Kits for the Detection of SARS-CoV-2. J. Med. Virol. 2021, 93, 3219–3226. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, N.; Noureen, N.; Faisal, A.; Zaheer, M.; Imran, M.; Ahsan, A.; Munir, R.; Zaidi, N. Factors Associated with Cycle Threshold Values (Ct-Values) of SARS-CoV2-RRT-PCR. Mol. Biol. Rep. 2022, 1–6. [Google Scholar] [CrossRef]
- Walker, A.S.; Pritchard, E.; House, T.; Robotham, J.V.; Birrell, P.J.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. CT Threshold Values, a Proxy for Viral Load in Community Sars-Cov-2 Cases, Demonstrate Wide Variation across Populations and over Time. eLife 2021, 10, e64683. [Google Scholar] [CrossRef]
- Bourouiba, L.; Dehandschoewercker, E.; Bush, J.W.M. Violent Expiratory Events: On Coughing and Sneezing. J. Fluid Mech. 2014, 745, 537–563. [Google Scholar] [CrossRef]
- Karlsson, J.A.; Sant’Ambrogio, G.; Widdicombe, J. Afferent Neural Pathways in Cough and Reflex Bronchoconstriction. J. Appl. Physiol. 1988, 65, 1007–1023. [Google Scholar] [CrossRef]
- Duguid, J.P. The Size and the Duration of Air-Carriage of Respiratory Droplets and Droplet-Nuclei. J. Hyg. 1946, 44, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Stadnytskyi, V.; Anfinrud, P.; Bax, A. Breathing, Speaking, Coughing or Sneezing: What Drives Transmission of SARS-CoV-2? J. Intern. Med. 2021, 290, 1010–1027. [Google Scholar] [CrossRef]
- Madas, B.G.; Füri, P.; Farkas, Á.; Nagy, A.; Czitrovszky, A.; Balásházy, I.; Schay, G.G.; Horváth, A. Deposition Distribution of the New Coronavirus (SARS-CoV-2) in the Human Airways upon Exposure to Cough-Generated Droplets and Aerosol Particles. Sci. Rep. 2020, 10, 22430. [Google Scholar] [CrossRef]
- Viklund, E.; Kokelj, S.; Larsson, P.; Nordén, R.; Andersson, M.; Beck, O.; Westin, J.; Olin, A.C. Severe Acute Respiratory Syndrome Coronavirus 2 Can Be Detected in Exhaled Aerosol Sampled during a Few Minutes of Breathing or Coughing. Influenza Other Respi. Viruses 2022, 16, 402–410. [Google Scholar] [CrossRef]
- Singanayagam, A.; Patel, M.; Charlett, A.; Bernal, J.L.; Saliba, V.; Ellis, J.; Ladhani, S.; Zambon, M.; Gopal, R. Duration of Infectiousness and Correlation with RT-PCR Cycle Threshold Values in Cases of COVID-19, England, January to May 2020. Eurosurveillance 2020, 25, 2001483. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Tirupathi, R.; Sule, A.A.; Aldali, J.; Mutair, A.; Alhumaid, S.; Muzaheed; Gupta, N.; Koritala, T.; Adhikari, R.; et al. Viral Dynamics and Real-Time Rt-Pcr Ct Values Correlation with Disease Severity in COVID-19. Diagnostics 2021, 11, 1091. [Google Scholar] [CrossRef]
- Abdulrahman, A.; Mallah, S.I.; Alawadhi, A.; Perna, S.; Janahi, E.M.; Alqahtani, M.M. Association between Rt-Pcr Ct Values and Covid-19 New Daily Cases: A Multicenter Cross-Sectional Study. Infez. Med. 2021, 29, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Scientific Brief: SARS-CoV-2 Transmission. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.htmle-briefs%2Fscientific-brief-sars-cov-2.html (accessed on 5 February 2022).
- Furuse, Y.; Sando, E.; Tsuchiya, N.; Miyahara, R.; Yasuda, I.; Ko, Y.K.; Saito, M.; Morimoto, K.; Imamura, T.; Shobugawa, Y.; et al. Clusters of Coronavirus Disease in Communities, Japan, January-April 2020. Emerg. Infect. Dis. 2020, 26, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Hyun, M.; Seok, J.; Hyuck, J.; Sung, Y.; Hyun, D.; Han, S.; Cho, C. Epidemiological and Clinical Characteristics of Coronavirus Disease 2019 in Daegu, South Korea. Int. J. Infect. Dis. 2020, 98, 462–466. [Google Scholar]
- Tsou, T.P.; Chen, W.C.; Huang, A.S.E.; Chang, S.C.; Su, C.P.; Lee, P.H.; Chan, P.C.; Wu, H.H.; Huang, S.T.; Su, W.J.; et al. Epidemiology of the First 100 Cases of COVID-19 in Taiwan and Its Implications on Outbreak Control. J. Formos. Med. Assoc. 2020, 119, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.S.; Lee, S.S.; Kwan, T.H.; Yeoh, E.K. Settings of Virus Exposure and Their Implications in the Propagation of Transmission Networks in a COVID-19 Outbreak. Lancet Reg. Health West. Pacific 2020, 4, 100052. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, J.; Wang, X.; Yang, J.; Liu, X.F.; Xu, X.K. Characterizing COVID-19 Transmission: Incubation Period, Reproduction Rate, and Multiple-Generation Spreading. Front. Phys. 2021, 8, 588. [Google Scholar] [CrossRef]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal Dynamics in Viral Shedding and Transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [Green Version]
- Woolhouse, M.E.J.; Dye, C.; Etard, J.-F.; Smith, T.; Charlwood, J.D.; Garnett, P.; Hagan, P.; Hii, J.L.K.; Ndhlovu, P.D.; Quinnell, R.J.; et al. Heterogeneities in the Transmission of Infectious Agents: Implications for the Design of Control Programs. Proc. Natl. Acad. Sci. USA 1997, 94, 338–342. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Wang, W.; Gao, L.; Wang, Y.; Luo, K.; Ren, L.; Zhan, Z.; Chen, X.; Zhao, S.; Huang, Y.; et al. Transmission Heterogeneities, Kinetics, and Controllability of SARS-CoV-2. Science 2021, 371, eabe2424. [Google Scholar] [CrossRef]
- Koopman, J. Modeling Infection Transmission. Annu. Rev. Public Health 2004, 25, 303–326. [Google Scholar] [CrossRef]
- Becker, N.G.; Britton, T. Statistical Studies of Infectious Disease Incidence. J. R. Stat. Soc. Ser. B Stat. Methodol. 1999, 61, 287–307. [Google Scholar] [CrossRef]
- Ahmed, E.I.; Zehr, J.L.; Schulz, K.M.; Lorenz, B.H.; DonCarlos, L.L.; Sisk, C.L. Transmission Dynamics and Control of Severe Acute Respiratory Syndrome. PLoS ONE 2008, 32, 736–740. [Google Scholar] [CrossRef]
- Illingworth, C.; Hamilton, W.L.; Warne, B.; Routledge, M.; Popay, A.; Jackson, C.; Fieldman, T.; Meredith, L.W.; Houldcroft, C.J.; Hosmillo, M.; et al. Superspreaders Drive the Largest Outbreaks of Hospital Onset Covid-19 Infections. eLife 2021, 10, e67308. [Google Scholar] [CrossRef]
- Cave, E. COVID-19 Super-Spreaders: Definitional Quandaries and Implications. Asian Bioeth. Rev. 2020, 12, 235–242. [Google Scholar] [CrossRef]
- Lu, J.G.; Jin, P.; English, A.S. Collectivism Predicts Mask Use during COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2021793118. [Google Scholar] [CrossRef]
- Dye, C.; Gay, N. Modeling the SARS Epidemic. Science 2003, 300, 1884–1885. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.Z.; Koopmans, M.; Fisman, D.N.; Gu, F.X. Understanding Why Superspreading Drives the COVID-19 Pandemic but Not the H1N1 Pandemic. Lancet Infect. Dis. 2021, 21, 1203–1204. [Google Scholar] [CrossRef]
- Celis, N.; Via, A.F.P.; Suggest, S. Wuhan Market Was Epidentre of Pandemic’s. Nat. Lett. 2022, 15–17. [Google Scholar]
- Gao, G.; Liu, W.; Wong, G.; Wang, J.; Wang, F.; Li, M. Surveillance of SARS-CoV-2 in the Environment and Animal Samples of the Huanan Seafood Market. Biol. Sci. 2022. [Google Scholar] [CrossRef]
- Leclerc, Q.J.; Fuller, N.M.; Knight, L.E.; Funk, S.; Knight, G.M. What Settings Have Been Linked to SARS-CoV-2 Transmission Clusters? Wellcome Open Res. 2020, 5, 83. [Google Scholar] [CrossRef]
- Beldomenico, P.M. Do Superspreaders Generate New Superspreaders? A Hypothesis to Explain the Propagation Pattern of COVID-19. Int. J. Infect. Dis. 2020, 96, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.A. Super-Spreaders in Infectious Diseases. Int. J. Infect. Dis. 2011, 15, e510–e513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, D.A.; Ausiello, D.; Salzman, J.; Devlin, T.; Langer, R.; Beddingfield, B.J.; Fears, A.C.; Doyle-Meyers, L.A.; Redmann, R.K.; Killeen, S.Z.; et al. Exhaled Aerosol Increases with COVID-19 Infection, Age, and Obesity. Proc. Natl. Acad. Sci. USA 2021, 118, e2021830118. [Google Scholar] [CrossRef] [PubMed]
- Majra, D.; Benson, J.; Pitts, J.; Stebbing, J. SARS-CoV-2 (COVID-19) Superspreader Events. J. Infect. 2021, 82, 36–40. [Google Scholar] [CrossRef]
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV Viral Load Dynamics, Duration of Viral Shedding, and Infectiousness: A Systematic Review and Meta-Analysis. Lancet Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef]
- Fain, B.; Dobrovolny, H.M. Initial Inoculum and the Severity of COVID-19: A Mathematical Modeling Study of the Dose–response of SARS-CoV-2 Infections. Epidemiologia 2020, 1, 5–15. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J. Dose–response Relation Deduced for Coronaviruses from COVID-19, SARS and MERS Meta-Analysis Results and Its Application for Infection Risk Assessment of Aerosol Transmission. Oxford Univ. Press Infect. Dis. Soc. Am. 2020. [Google Scholar]
- Therese, S.S.; Julian, D.; Bianca, J.; Carmen, S.; Sarah, G.; Stephan, K.; Christian, S.; Wilfried, P.; Gernot, W. An in Vitro Model for Assessment of SARS-CoV-2 Infectivity by Defining the Correlation between Virus Isolation and Quantitative PCR Value: Isolation Success of SARS-CoV-2 from Oropharyngeal Swabs Correlates Negatively with Cq Value. Virol. J. 2021, 18, 1–7. [Google Scholar] [CrossRef]
- Killingley, B.; Mann, A.; Kalinova, M.; Hare, S.S.; Brown, J.; Harden, S. Safety, Tolerability and Viral Kinetics during SARS-CoV-2 Human Challenge. Nat. Med. 2022. [Google Scholar] [CrossRef]
- Bao, L.; Gao, H.; Deng, W.; Lv, Q.; Yu, H.; Liu, M.; Yu, P.; Liu, J.; Qu, Y.; Gong, S.; et al. Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 via Close Contact and Respiratory Droplets among Human Angiotensin-Converting Enzyme 2 Mice. J. Infect. Dis. 2020, 222, 551–555. [Google Scholar] [CrossRef]
- Ryan, K.A.; Bewley, K.R.; Fotheringham, S.A.; Slack, G.S.; Brown, P.; Hall, Y.; Wand, N.I.; Marriott, A.C.; Cavell, B.E.; Tree, J.A.; et al. Dose-Dependent Response to Infection with SARS-CoV-2 in the Ferret Model and Evidence of Protective Immunity. Nat. Commun. 2021, 12, 81. [Google Scholar] [CrossRef]
- Cross, R.W.; Agans, K.N.; Prasad, A.N.; Borisevich, V.; Woolsey, C.; Deer, D.J.; Dobias, N.S.; Geisbert, J.B.; Fenton, K.A.; Geisbert, T.W. Intranasal Exposure of African Green Monkeys to SARS-CoV-2 Results in Acute Phase Pneumonia with Shedding and Lung Injury Still Present in the Early Convalescence Phase. Virol. J. 2020, 17, 125. [Google Scholar] [CrossRef]
- Bao, L.; Deng, W.; Huang, B.; Gao, H.; Liu, J.; Ren, L.; Wei, Q.; Yu, P.; Xu, Y.; Qi, F.; et al. The Pathogenicity of SARS-CoV-2 in HACE2 Transgenic Mice. Nature 2020, 583, 830–833. [Google Scholar] [CrossRef]
- Schlottau, K.; Rissmann, M.; Graaf, A.; Schön, J.; Sehl, J.; Wylezich, C.; Höper, D.; Mettenleiter, T.C.; Balkema-Buschmann, A.; Harder, T.; et al. SARS-CoV-2 in Fruit Bats, Ferrets, Pigs, and Chickens: An Experimental Transmission Study. Lancet Microbe 2020, 1, e218–e225. [Google Scholar] [CrossRef]
- Brouwer, A.F.; Weir, M.H.; Eisenberg, M.C.; Meza, R.; Eisenberg, J.N.S. Dose–response Relationships for Environmentally Mediated Infectious Disease Transmission Models. PLoS Comput. Biol. 2017, 13, e1005481. [Google Scholar] [CrossRef] [Green Version]
- Haas, N.; Rose, J.B.; Gerba, P.G. Quantitative Microbial Risk Assessment, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Lee, J.; Yoo, D.; Ryu, S.; Ham, S.; Lee, K.; Yeo, M.; Min, K.; Yoon, C. Quantity, Size Distribution, and Characteristics of Cough-Generated Aerosol Produced by Patients with an Upper Respiratory Tract Infection. Aerosol Air Qual. Res. 2019, 19, 840–853. [Google Scholar] [CrossRef]
- Shim, E.; Tariq, A.; Choi, W.; Lee, Y.; Chowell, G. Transmission Potential and Severity of COVID-19 in South Korea. Int. J. Infect. Dis. 2020, 93, 339–344. [Google Scholar] [CrossRef]
- Zauzmer, J. ‘Take It Very Seriously’: Pastor at Arkansas Church Where 34 People Came down with Coronavirus Sends a Warning. The Washington Post. 2020. Available online: http://web.archive.org/web/20200420073657/https://www.washingtonpost.com/religion/2020/03/24/pastor-arkansas-church-coronavirus-warning-greers-ferry/ (accessed on 1 March 2022).
- McKie, R. Did Singing Together Spread Coronavirus to Four Choirs? The Observer. 2020. Available online: https://www.theguardian.com/world/2020/may/17/did-singing-together-spread-coronavirus-to-four-choirs (accessed on 1 March 2022).
- Singapore Upcode Academy. Dashboard of the COVID-19 Virus Outbreak in Singapore. 2021. Available online: http://web.archive.org/web/20200420073524/https://www.againstcovid19.com/singapore?start=21-01-2020&end=20-04-2020 (accessed on 1 March 2022).
- Adam, D.C.; Wu, P.; Wong, J.Y.; Lau, E.H.Y.; Tsang, T.K.; Cauchemez, S.; Leung, G.M.; Cowling, B.J. Clustering and Superspreading Potential of SARS-CoV-2 Infections in Hong Kong. Nat. Med. 2020, 26, 1714–1719. [Google Scholar] [CrossRef]
- DW. Coronavirus: German Slaughterhouse Outbreak Crosses. DW News. 2020. Available online: https://www.dw.com/en/coronavirus-german-slaughterhouse-outbreak-crosses-1000/a-53883372 (accessed on 1 March 2022).
- Cannon, A. Spike in COVID-19 cases in Iowa packing plants a big part of 389 new cases, state’s largest single-day increase. Des Moines Register. 2020. Available online: https://eu.desmoinesregister.com/story/news/2020/04/19/coronavirus-iowa-largest-single-day-increase-iowa-covid-19-cases-tied-meatpacking-plants/5162127002/ (accessed on 1 March 2022).
- Adebayo, B. A Worker Infected 533 Others With Coronavirus at a Factory in Ghana, President Says. CNN. 2020. Available online: https://edition.cnn.com/2020/05/11/africa/ghana-factory-coronavirus-infection-intl/index.html (accessed on 1 March 2022).
- Halliday, J. Over 450 Covid-19 Cases Reported at Food Factories in England and Wales. The Guardian. 2020. Available online: https://www.theguardian.com/uk-news/2020/jun/25/over-450-covid-19-cases-reported-at-food-factories-in-england-and-wales (accessed on 1 March 2022).
- Park, S.Y.; Kim, Y.M.; Yi, S.; Lee, S.; Na, B.J.; Kim, C.B.; Kim, J.I.; Kim, H.S.; Kim, Y.B.; Park, Y.; et al. Coronavirus Disease Outbreak in Call Center, South Korea. Emerg. Infect. Dis. 2020, 26, 1666–1670. [Google Scholar] [CrossRef]
- Fontanet, A.; Tondeur, L.; Madec, Y.; Grant, R.; Besombes, C.; Jolly, N.; Pellerin, S.F.; Ungeheuer, M.-N.; Cailleau, I.; Kuhmel, L.; et al. Cluster of COVID-19 in Northern France: A Retrospective Closed Cohort Study. SSRN Electron. J. 2020, 1–22. [Google Scholar] [CrossRef]
- Kadari-Ovadia, S.; Hasson, N.; Efrati, I. Schools in Jerusalem Shut as Dozens of Students, Staff Test Positive for the Coronavirus. Haaretz, Israel News. 2020. Available online: https://www.haaretz.com/israel-news/.premium-half-of-new-coronavirus-cases-in-schools-came-from-single-school-in-jerusalem-1.8885755 (accessed on 1 March 2022).
- Ministry of Health of New Zeland. COVID-19—Significant Clusters. 2020. Available online: https://www.health.govt.nz/our-work/diseases-and-conditions/covid-19-novel-coronavirus/covid-19-current-situation/covid-19-current-cases/covid-19-significant-clusters (accessed on 1 March 2022).
- Singapore Government Agency. News Highlights. Singapore Government Agency Website. 2020. Available online: http://web.archive.org/web/20200420074230/https://www.moh.gov.sg/news-highlights/details/16-more-cases-discharged-35-new-cases-of-covid-19-infection-confirmed (accessed on 1 March 2022).
- Cai, J.; Sun, W.; Huang, J.; Gamber, M.; Wu, J.; He, G. Indirect Virus Transmission in Cluster of COVID-19 Cases, Wenzhou, China, 2020. Emerg. Infect. Dis. 2020, 26, 1343–1345. [Google Scholar] [CrossRef]
- Chu, J.; Yang, N.; Wei, Y.; Yue, H.; Zhang, F.; Zhao, J.; He, L.; Sheng, G.; Chen, P.; Li, G.; et al. Clinical Characteristics of 54 Medical Staff with COVID-19: A Retrospective Study in a Single Center in Wuhan, China. J. Med. Virol. 2020, 92, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Sim, W. Japan Identifies 15 Clusters as Covid-19 Cases. The Straits Times. 2020. Available online: http://web.archive.org/web/20200420073241/https://www.straitstimes.com/asia/east-asia/japan-identifies-15-clusters-as-covid-19-cases-mount (accessed on 1 March 2022).
- A.T.S. «Superspreader»: 300 Visiteurs d’un Club Zurichois En Quarantaine. Le Temps. 2020. Available online: https://www.letemps.ch/suisse/superspreader-300-visiteurs-dun-club-zurichois-quarantaine?utm_source=POLITICO.EU&utm_campaign=4cfd6ca4cf-EMAIL_CAMPAIGN_2020_06_28_03_00&utm_medium=email&utm_term=0_10959edeb5-4cfd6ca4cf-190608896 (accessed on 1 March 2022).
- Stuff. Coronavirus: Matamata Bar Owner on NZ’s Biggest Covid-19 Cluster Outbreak. Stuff. 2020. Available online: https://www.stuff.co.nz/national/health/coronavirus/121462144/coronavirus-matamata-bar-owner-on-nzs-biggest-covid19-cluster-outbreak (accessed on 1 March 2022).
- Al Jazeera News. After One Infected 16 at Berlin Nightclub, Coronavirus Fears Grow. Al Jazeera News. 2020. Available online: http://web.archive.org/web/20200420071353/https://www.aljazeera.com/news/2020/03/infected-16-berlin-nightclub-coronavirus-fears-grow-200310132859234.html (accessed on 1 March 2022).
- CNN. How an Austrian Ski Resort Helped Coronavirus Spread Across Europe. CNN News. 2020. Available online: https://edition.cnn.com/2020/03/24/europe/austria-ski-resort-ischgl-coronavirus-intl/ (accessed on 1 March 2022).
- Marcelo, P.; O’brien, M. Cluster of Coronavirus Cases Tied to U.S. Biotech Meeting. Al Jazeera News. 2020. Available online: http://web.archive.org/web/20200401222019/https://time.com/5801554/coronavirus-cluster-biotech-biogen-boston-cambridge/ (accessed on 1 March 2022).
- Liu, Y.; Eggo, R.M.; Kucharski, A.J. Secondary Attack Rate and Superspreading Events for SARS-CoV-2. Lancet 2020, 365, e47. [Google Scholar] [CrossRef] [Green Version]
- Orban, A. 26 Passengers Tested Positive for Covid-19 on an Emirates Flight to Hong Kong. Aviation 24 Website. 2020. Available online: https://www.aviation24.be/airlines/emirates-airline/26-passengers-tested-positive-on-an-emirates-flight-to-hong-kong/ (accessed on 1 March 2022).
- Shen, Y.; Li, C.; Dong, H.; Wang, Z.; Martinez, L.; Sun, Z.; Handel, A.; Chen, Z.; Chen, E.; Ebell, M.H.; et al. Community Outbreak Investigation of SARS-CoV-2 Transmission among Bus Riders in Eastern China. JAMA Intern. Med. 2020, 180, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Kim, S.G.; Kim, S.M.; Kim, E.H.; Park, S.J.; Yu, K.M.; Chang, J.H.; Kim, E.J.; Lee, S.; Casel, M.A.B.; et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe 2020, 27, 704–709. [Google Scholar] [CrossRef]
- Richard, M.; Kok, A.; De Meulder, D.; Bestebroer, T.M.; Lamers, M.M.; Okba, N.M.A.; Van Vlissingen, M.F.; Rockx, B.; Haagmans, B.L.; Koopmans, M.P.G.; et al. SARS-CoV-2 Is Transmitted via Contact and via the Air between Ferrets. Nat. Commun. 2020, 11, 3496. [Google Scholar] [CrossRef]
- Sun, S.H.; Chen, Q.; Gu, H.J.; Yang, G.; Wang, Y.X.; Huang, X.Y.; Liu, S.S.; Zhang, N.N.; Li, X.F.; Xiong, R.; et al. A Mouse Model of SARS-CoV-2 Infection and Pathogenesis. Cell Host Microbe 2020, 28, 124–133. [Google Scholar] [CrossRef]
- Di Jiang, R.; Liu, M.Q.; Chen, Y.; Shan, C.; Zhou, Y.W.; Shen, X.R.; Li, Q.; Zhang, L.; Zhu, Y.; Si, H.R.; et al. Pathogenesis of SARS-CoV-2 in Transgenic Mice Expressing Human Angiotensin-Converting Enzyme 2. Cell 2020, 182, 50–58. [Google Scholar] [CrossRef]
- Dinnon, H.; Leist, S.; Schäfer, A.; Edwards, C.; Martinez, D.; Montgomery, S.; West, A.; Yount, B.; Hou, Y.; Adams, L.; et al. A Mouse-Adapted SARS-CoV-2 Model for the Evaluation of COVID-19 Medical Countermeasures. bioRxiv 2020. [Google Scholar] [CrossRef]
- Rockx, B.; Kuiken, T.; Herfst, S.; Bestebroer, T.; Lamers, M.M.; Munnink, B.B.O.; De Meulder, D.; Van Amerongen, G.; Van Den Brand, J.; Okba, N.M.A.; et al. Comparative Pathogenesis of COVID-19, MERS, and SARS in a Nonhuman Primate Model. Science 2020, 368, 1012–1015. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Bao, L.; Gao, H.; Xiang, Z.; Qu, Y.; Song, Z.; Gong, S.; Liu, J.; Liu, J.; Yu, P.; et al. Ocular Conjunctival Inoculation of SARS-CoV-2 Can Cause Mild COVID-19 in Rhesus Macaques. Nat. Commun. 2020, 11, 4400. [Google Scholar] [CrossRef]
- Munster, V.J.; Feldmann, F.; Williamson, B.N.; van Doremalen, N.; Pérez-Pérez, L.; Schulz, J.; Meade-White, K.; Okumura, A.; Callison, J.; Brumbaugh, B.; et al. Respiratory Disease and Virus Shedding in Rhesus Macaques Inoculated with SARS-CoV-2 1. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Chandrashekar, A.; Liu, J.; Martino, A.J.; McMahan, K.; Mercad, N.B.; Peter, L.; Tostanosk, L.H.; Yu, J.; Maliga, Z.; Nekorchuk, M.; et al. SARS-CoV-2 Infection Protects against Rechallenge in Rhesus Macaques. Science 2020, 369, 812–817. [Google Scholar] [CrossRef]
- Johnston, S.C.; Ricks, K.M.; Jay, A.; Raymond, J.L.; Rossi, F.; Zeng, X.; Scruggs, J.; Dyer, D.; Frick, O.; Koehler, J.W.; et al. Development of a Coronavirus Disease 2019 Nonhuman Primate Model Using Airborne Exposure. bioRxiv 2021, arXiv:0246366. [Google Scholar] [CrossRef]
- Woolsey, C.; Borisevich, V.; Prasad, A.N.; Agans, K.N.; Deer, D.J.; Dobias, N.S.; Heymann, J.C.; Foster, S.L.; Levine, C.B.; Medina, L.; et al. Establishment of an African Green Monkey Model for COVID-19 and Protection against Re-Infection. Nat. Immunol. 2021, 22, 86–98. [Google Scholar] [CrossRef]
- Blair, R.; Vaccari, M.; Doyle, L.; Roy, C.; Russell, K.; Fahlberg, M.; Monjure, C.; Beddingfield, B.; Plante, K.; Plante, K.; et al. ARDS and Cytokine Storm in SARS-CoV-2 Infected Caribbean Vervets. bioRxiv 2021, arXiv:157933. [Google Scholar] [CrossRef]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian Hamsters as a Small Animal Model for SARS-CoV-2 Infection and Countermeasure Development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef]
- Osterrieder, N.; Bertzbach, L.D.; Dietert, K.; Abdelgawad, A. Age-Dependent Progression of SARS-CoV-2 Infection. Viruses 2020, 12, 779. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Bhopal, R.; Huy, N.T. Review of Infective Dose, Routes of Transmission, and Outcome of COVID-19 Caused by the SARS-COV-2: Comparison with Other Respiratory Viruses. Epidemiol. Infect. 2021, 149, e96. [Google Scholar] [CrossRef]
- Danis, K.; Epaulard, O.; Bénet, T.; Gaymard, A.; Campoy, S.; Botelho-Nevers, E.; Bouscambert-Duchamp, M.; Spaccaferri, G.; Ader, F.; Mailles, A.; et al. Cluster of coronavirus disease 2019 (COVID-19) in the French Alps, February 2020. Clin. Infect. Dis. 2020, 71, 825–832. [Google Scholar] [CrossRef] [Green Version]



| Patient | Age | Gender | DASO * | Sampling Period | Initial Ct | Final Ct | Vaccines | Hospitalization | Group |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 59 | Male | 3 days | 1 day | 28.1 | N/A | 3 | Yes | A |
| Patient 2 | 45 | Female | 2 days | 1 day | 29.8 | N/A | 3 | Yes | A |
| Patient 3 | 59 | Male | 2 days | 3 days | 19.3 | 30.4 | 3 1 | No | A |
| Patient 4 | 22 | Male | 1 day | 4 days | 23.7 | 36.1 | 2 | No | A |
| Patient 5 | 26 | Male | 1 day | 3 days | 23.2 | 29.3 | 2 | No | A |
| Patient 6 | 89 | Male | 0 days † | 1 day | 16.7 | N/A | 3 | Yes | B |
| Patient 7 | 75 | Male | 0 days † | 1 day | 16.8 | N/A | 3 | Yes | B |
| Patient 8 | 61 | Male | 0 days † | 1 day | 13.5 | N/A | 3 | Yes | B |
| Patient 9 | 59 | Female | 1 days † | 1 day | 32.7 | N/A | No | Yes | B |
| Patient 10 | 84 | Male | 2 days † | 1 day | 32.8 | N/A | 3 | Yes | B |
| Patient 11 | 63 | Male | 0 days † | 1 day | 33.1 | N/A | 3 | Yes | B |
| Patient 12 | 89 | Male | 0 days † | 1 day | 28.8 | N/A | 3 | Yes | B |
| Patient 13 | 68 | Male | 1 day † | 1 day | 24.8 | N/A | 3 | Yes | B |
| Patient 14 | 69 | Female | 6 days † | 1 day | 26.1 | N/A | No | Yes | B |
| Patient 15 | 88 | Female | 1 day † | 1 day | 34.8 | N/A | 3 | Yes | B |
| Patient 16 | 93 | Male | 1 day † | 1 day | 28.8 | N/A | 3 | Yes | B |
| Patient 17 | 88 | Male | 1 day † | 1 day | 33.3 | N/A | 3 | Yes | B |
| Patient 18 | 54 | Female | 1 day † | 1 day | 21.8 | N/A | 3 | Yes | B |
| Patient 19 | 67 | Male | 7 days †† | 1 day | 15.8 | N/A | 3 | Yes | B |
| Patient 20 | 22 | Female | 2 days | 2 days | 27.1 | 29.2 | 2 | No | C |
| Patient 21 | 49 | Female | 2 days | 1 day | 29.3 | N/A | 2 | No | C |
| Patient 22 | 14 | Female | 2 days | 1 day | 21.2 | N/A | 2 | No | C |
| Sample | Amplification | Day 2 | Day 3 | Day 4 | Day 6 | Day 7 |
|---|---|---|---|---|---|---|
| Nasopharynx | pan-SARS ESAR | 25.5 (5.5 × 106 copies/mL) | 23.8 (2.0 × 107 copies/mL) | 25.9 (4.8 × 106 copies/mL) | 33.8 (2.3 × 104 copies/mL) | 36.1 (4.9 × 103 copies/mL) |
| SARS-CoV-2 IP4 | 24.6 (3.2 × 106 copies/mL) | 23.1 (2.5 × 107 copies/mL) | 25.4 (5.4 × 106 copies/mL) | 34.4 (1.2 × 104 copies/mL) | Not detected | |
| Oropharyngea | pan-SARS ESAR | 29.1 (4.8 × 105 copies/mL) | 29.3 (4.8 × 105 copies/mL) | Not detected | Not detected | Not detected |
| SARS-CoV-2 IP4 | 28.9 (3.2 × 105 copies/mL) | 29.4 (3.7 × 105 copies/mL) | Not detected | Not detected | Not detected | |
| Coughs | pan-SARS ESAR | 28.5 (7.3 × 105 copies/mL) | Not detected | Not detected | Not detected | Not detected |
| SARS-CoV-2 IP4 | 27.4 (9.1 × 105 copies/mL) | Not detected | Not detected | Not detected | Not detected |
| Sample | Amplification | Day 2 | Day 3 | Day 4 |
|---|---|---|---|---|
| Nasopharynx | pan-SARS ESAR | 25.1 (7.8 × 106 copies/mL) | 23.3 (2.7 × 107 copies/mL) | 28.7 (6.7 × 105 copies/mL) |
| SARS-CoV-2 IP4 | 24.6 (8.9 × 106 copies/mL) | 22.9 (2.8 × 107 copies/mL) | 28.3 (7.4 × 105 copies/mL) | |
| Oropharyngeal | pan-SARS ESAR | Not detected | Not detected | Not detected |
| SARS-CoV-2 IP4 | Not detected | Not detected | Not detected | |
| Coughs | pan-SARS ESAR | Not detected | Not detected | Not detected |
| SARS-CoV-2 IP4 | Not detected | Not detected | Not detected |
| Sample | CO2 Levels | Amplification | Patient 20 |
|---|---|---|---|
| Day 1 | |||
| 1 | 500–1000 ppm | pan-SARS ESAR | 36.7 (1.1 × 103 copies/mL) |
| SARS-CoV-2 IP4 | Not detected | ||
| 2 | 500–1000 ppm | pan-SARS ESAR | 36.6 (2.3 × 103 copies/mL) |
| SARS-CoV-2 IP4 | 36.8 (2.0 × 103 copies/mL) | ||
| 3 | 500–1000 ppm | pan-SARS ESAR | 37.2 (1.6 × 103 copies/mL) |
| SARS-CoV-2 IP4 | Not detected | ||
| 4 | 500–1000 ppm | pan-SARS ESAR | 38.9 (Suspicious) |
| SARS-CoV-2 IP4 | 38.8 (Suspicious) | ||
| 5 | 500–1000 ppm | pan-SARS ESAR | Not detected |
| SARS-CoV-2 IP4 | 38.7 (Suspicious) | ||
| 6 | 500–1000 ppm | pan-SARS ESAR | 37.9 (9.6 × 102 copies/mL) |
| SARS-CoV-2 IP4 | Not detected | ||
| 7 | 1000–1500 ppm | pan-SARS ESAR | 37.2 (1.5 × 103 copies/mL) |
| SARS-CoV-2 IP4 | 39.2 (Suspicious) | ||
| 8 | 1000–1500 ppm | pan-SARS ESAR | 38.8 (Suspicious) |
| SARS-CoV-2 IP4 | 38.3 (Suspicious) | ||
| 9 | 1000–1500 ppm | pan-SARS ESAR | 39.8 (Suspicious) |
| SARS-CoV-2 IP4 | Not detected | ||
| 10 | 1500–2000 ppm | pan-SARS ESAR | 35.5 (4.8 × 103 copies/mL) |
| SARS-CoV-2 IP4 | 36.3 (Suspicious) | ||
| 11 | 1500–2000 ppm | pan-SARS ESAR | 37.0 (1.8 × 103 copies/mL) |
| SARS-CoV-2 IP4 | 38.3 (Suspicious) | ||
| Patient 20–22 | |||
| Day 3 | |||
| 12 | 500–1000 ppm | pan-SARS ESAR | 39.0 (Suspicious) |
| SARS-CoV-2 IP4 | Not detected | ||
| 13 | 1000–1500 ppm | pan-SARS ESAR | Not detected |
| SARS-CoV-2 IP4 | 38.9 (Suspicious) | ||
| 14 | 1500–2000 ppm | pan-SARS ESAR | 39.0 (Suspicious) |
| SARS-CoV-2 IP4 | Not detected | ||
| 15 | 1500–2000 ppm | pan-SARS ESAR | 39.5 (Suspicious) |
| SARS-CoV-2 IP4 | Not detected |
| Sample | Amplification | Day 2 | Day 4 | Day 6 |
|---|---|---|---|---|
| Nasopharynx | pan-SARS ESAR | 19.9 (1.3 × 107 copies/mL) | 30.8 (1.4 × 104 copies/mL) | 33.4 (2.5 × 104 copies/mL) |
| SARS-CoV-2 IP4 | 19.1 (2.5 × 107 copies/mL) | 30.6 (1.5 × 104 copies/mL) | 32.6 (3.7 × 104 copies/mL) | |
| Oropharyngeal | pan-SARS ESAR | 21.9 (3.7 × 106 copies/mL) | Not detected | 38.9 (Suspicious) |
| SARS-CoV-2 IP4 | 21.2 (5.9 × 106 copies/mL) | Not detected | 37.2 (1.6 × 103 copies/mL) | |
| Coughs | pan-SARS ESAR | Not detected | Not detected | Not detected |
| SARS-CoV-2 IP4 | Not detected | Not detected | Not detected |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baselga, M.; Güemes, A.; Alba, J.J.; Schuhmacher, A.J. SARS-CoV-2 Droplet and Airborne Transmission Heterogeneity. J. Clin. Med. 2022, 11, 2607. https://doi.org/10.3390/jcm11092607
Baselga M, Güemes A, Alba JJ, Schuhmacher AJ. SARS-CoV-2 Droplet and Airborne Transmission Heterogeneity. Journal of Clinical Medicine. 2022; 11(9):2607. https://doi.org/10.3390/jcm11092607
Chicago/Turabian StyleBaselga, Marta, Antonio Güemes, Juan J. Alba, and Alberto J. Schuhmacher. 2022. "SARS-CoV-2 Droplet and Airborne Transmission Heterogeneity" Journal of Clinical Medicine 11, no. 9: 2607. https://doi.org/10.3390/jcm11092607
APA StyleBaselga, M., Güemes, A., Alba, J. J., & Schuhmacher, A. J. (2022). SARS-CoV-2 Droplet and Airborne Transmission Heterogeneity. Journal of Clinical Medicine, 11(9), 2607. https://doi.org/10.3390/jcm11092607