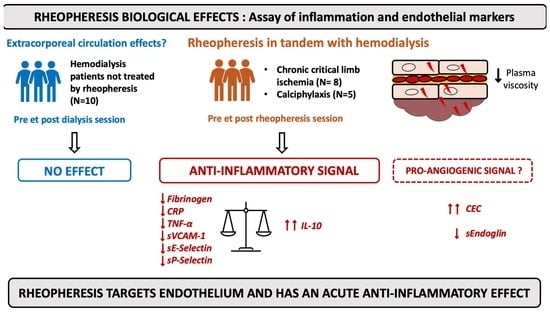
Rheopheresis Performed in Hemodialysis Patients Targets Endothelium and Has an Acute Anti-Inflammatory Effect
Abstract
:1. Introduction
2. Material and Methods
2.1. Population
2.2. Rheopheresis Treatment
2.3. Blood Assays
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Acute Effect of Hemodialysis Sessions on Inflammatory and Endothelial Markers
3.3. Acute Effect of Rheopheresis Sessions
3.4. Long-Term Effect of Rheopheresis Sessions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klingel, R.; Fassbender, C.; Fassbender, T.; Erdtracht, B.; Berrouschot, J. Rheopheresis: Rheologic, functional, and structural aspects. Ther. Apher. 2000, 4, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Brunner, R.; Widder, R.A.; Walter, P.; Borberg, H.; Oette, K. Change in Hemorrheological and biochemical parameters following membrane differential filtration. Int. J. Artif. Organs 1995, 18, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Jung, F. From hemorheology to microcirculation and regenerative medicine: Fåhraeus lecture 2009. Clin. Hemorheol. Microcirc. 2010, 45, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villain, C.; Metzger, M.; Combe, C.; Fouque, D.; Frimat, L.; Jacquelinet, C.; Laville, M.; Briançon, S.; Klein, J.; Schanstra, J.P.; et al. Prevalence of atheromatous and non-atheromatous cardiovascular disease by age in chronic kidney disease. Nephrol. Dial. Transpl. 2020, 35, 827–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballew, S.H.; Matsushita, K. Cardiovascular risk prediction in CKD. Semin. Nephrol. 2018, 38, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Jourde-Chiche, N.; Dou, L.; Cerini, C.; Dignat-George, F.; Brunet, P. Vascular Incompetence in dialysis patients-protein-bound uremic toxins and endothelial dysfunction: Vascular incompetence in dialysis patients. Semin. Dial. 2011, 24, 327–337. [Google Scholar] [CrossRef]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef]
- Dou, L.; Sallée, M.; Cerini, C.; Poitevin, S.; Gondouin, B.; Jourde-Chiche, N.; Fallague, K.; Brunet, P.; Calaf, R.; Dussol, B.; et al. The cardiovascular effect of the uremic solute indole-3 acetic acid. J. Am. Soc. Nephrol. 2015, 26, 876–887. [Google Scholar] [CrossRef] [Green Version]
- Jourde-Chiche, N.; Dou, L.; Sabatier, F.; Calaf, R.; Cerini, C.; Robert, S.; Camoin-Jau, L.; Charpiot, P.; Argiles, A.; Dignat-George, F.; et al. Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J. Thromb. Haemost. 2009, 7, 1576–1584. [Google Scholar] [CrossRef]
- Hung, S.-C.; Kuo, K.-L.; Huang, H.-L.; Lin, C.-C.; Tsai, T.-H.; Wang, C.-H.; Chen, J.-W.; Lin, S.-J.; Huang, P.-H.; Tarng, D.-C. Indoxyl sulfate suppresses endothelial progenitor cell–mediated neovascularization. Kidney Int. 2016, 89, 574–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimmel, P.L.; Phillips, T.M.; Simmens, S.J.; Peterson, R.A.; Weihs, K.L.; Alleyne, S.; Cruz, I.; Yanovski, J.A.; Veis, J.H. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998, 54, 236–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, P.-S.; Wu, M.-Y.; Chien, S.-W.; Wu, T.-K.; Liu, C.-S.; Hu, C.-Y.; Chang, H.-C.; Tsai Pai, M.-A. Elevated circulating levels of soluble CD-40 ligand in haemodialysis patients with symptomatic coronary heart disease. Nephrology 2008, 13, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Péquériaux, N.C.; Fijnheer, R.; Gemen, E.F.; Barendrecht, A.; Dekker, F.; Krediet, R.T.; Beutler, J.J.; Boeschoten, E.W.; Roest, M. Plasma concentration of von Willebrand factor predicts mortality in patients on chronic renal replacement therapy. Nephrol. Dial. Transplant. 2012, 27, 2452–2457. [Google Scholar] [CrossRef] [Green Version]
- Giordano, M.; De Feo, P.; Lucidi, P.; Depascale, E.; Giordano, G.; Infantone, L.; Zoccolo, A.M.; Castellino, P. Increased albumin and fibrinogen synthesis in hemodialysis patients with normal nutritional status. J. Am. Soc. Nephrol. 2001, 12, 349–354. [Google Scholar] [CrossRef]
- Segarra, A.; Chacón, P.; Martinez-Eyarre, C.; Argelaguer, X.; Vila, J.; Ruiz, P.; Fort, J.; Bartolomé, J.; Camps, J.; Moliner, E.; et al. Circulating levels of plasminogen activator inhibitor type-1, tissue plasminogen activator, and thrombomodulin in hemodialysis patients: Biochemical correlations and role as independent predictors of coronary artery stenosis. J. Am. Soc. Nephrol. 2001, 12, 1255–1263. [Google Scholar] [CrossRef]
- Gondouin, B.; Cerini, C.; Dou, L.; Sallée, M.; Duval-Sabatier, A.; Pletinck, A.; Calaf, R.; Lacroix, R.; Jourde-Chiche, N.; Poitevin, S. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 2013, 84, 733–744. [Google Scholar] [CrossRef] [Green Version]
- Baskin, E.; Duman, O.; Beşbaş, N.; Ozen, S. Hypercoagulopathy in a hemodialysis patient: Are elevations in factors VII and VIII effective? Nephron 1999, 83, 180. [Google Scholar] [CrossRef]
- Bonomini, M.; Reale, M.; Santarelli, P.; Stuard, S.; Settefrati, N.; Albertazzi, A. Serum levels of soluble adhesion molecules in chronic renal failure and dialysis patients. Nephron 1998, 79, 399–407. [Google Scholar] [CrossRef]
- Papayianni, A.; Alexopoulos, E.; Giamalis, P.; Gionanlis, L.; Belechri, A.; Koukoudis, P.; Memmos, D. Circulating levels of ICAM-1, VCAM-1, and MCP-1 are increased in haemodialysis patients: Association with inflammation, dyslipidaemia, and vascular events. Nephrol. Dial. Transplant. 2002, 17, 435–441. [Google Scholar] [CrossRef]
- Padmanabhan, A.; Connelly-Smith, L.; Aqui, N.; A Balogun, R.; Klingel, R.; Meyer, E.; Pham, H.P.; Schneiderman, J.; Witt, V.; Wu, Y.; et al. Guidelines on the use of therapeutic apheresis in clinical practice—Evidence-based approach from the Writing Committee of the American Society for apheresis: The eighth special issue. J. Clin. Apher. 2019, 34, 171–354. [Google Scholar] [CrossRef] [PubMed]
- Pulido, J.S.; Multicenter Investigation of Rheopheresis for AMD (MIRA-1) Study Group. Multicenter prospective, randomized, double-masked, placebo-controlled study of Rheopheresis to treat nonexudative age-related macular degeneration: Interim analysis. Trans. Am. Ophthalmol. Soc. 2002, 100, 85–106; discussion 106–107. [Google Scholar] [PubMed]
- Mösges, R.; Köberlein, J.; Heibges, A.; Erdtracht, B.; Klingel, R.; Lehmacher, W. Rheopheresis for idiopathic sudden hearing loss: Results from a large prospective, multicenter, randomized, controlled clinical trial. Eur. Arch. Otorhinolaryngol. 2009, 266, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Klingel, R.; Erdtracht, B.; Gauss, V.; Piazolo, A.; Mausfeld-Lafdhiya, P.; Diehm, C. Rheopheresis in patients with critical limb ischemia—Results of an open label prospective pilot trial. Therapher. Dial. 2005, 9, 473–481. [Google Scholar] [CrossRef]
- Ferrannini, M.; Vischini, G.; Staffolani, E.; Scaccia, F.; Miani, N.; Parravano, M.; Louis, M.; Splendiani, G.; Di Daniele, N. Rheopheresis in vascular diseases. Int. J. Artif. Organs 2007, 30, 923–929. [Google Scholar] [CrossRef]
- Weiss, N. Lipid apheresis and rheopheresis for treatment of peripheral arterial disease. Atheroscler. Suppl. 2009, 10, 62–69. [Google Scholar] [CrossRef]
- Robert, T.; Lionet, A.; Bataille, S.; Seret, G. Rheopheresis: A new therapeutic approach in severe calciphylaxis. Nephrology 2019, 25, 298–304. [Google Scholar] [CrossRef]
- Solignac, J.; Bataille, S.; Touzot, M.; Bruner, F.; Bouchouareb, D.; Brunet, P.; Ridel, C.; Robert, T. Rheopheresis for severe peripheral arterial disease in hemodialysis patients: A clinical series. J. Clin. Apher. 2022, 37, 91–99. [Google Scholar] [CrossRef]
- Rossi, M.; Puccini, R.; Romagnoli, M.C.; Di Maria, C.; Mattei, P.; Bernini, M.; Marconcini, C.; Santoro, G. Acute and subacute effect of rheopheresis on microvascular endothelial function in patients suffering from age-related macular degeneration. Ther. Apher. Dial. 2009, 13, 540–548. [Google Scholar] [CrossRef]
- Balletshofer, B.M.; Stock, J.; Rittig, K.; Lehn-Stefan, A.; Braun, N.; Burkart, F.; Plontke, S.; Klingel, R.; Häring, H.-U. Acute effect of rheopheresis on peripheral endothelial dysfunction in patients suffering from sudden hearing loss. Ther. Apher. Dial. 2005, 9, 385–390. [Google Scholar] [CrossRef]
- Woywodt, A.; Blann, A.D.; Kirsch, T.; Erdbruegger, U.; Banzet, N.; Haubitz, M.; Dignat-George, F. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: Proposal of a definition and a consensus protocol. J. Thromb. Haemost. 2006, 4, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Van Beaumont, W. Evaluation of hemoconcentration from hematocrit measurements. J. Appl. Physiol. 1972, 32, 712–713. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. The proposal to lower p value thresholds to 0.005. JAMA 2018, 319, 1429. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, R. P values, the ‘gold standard’ of statistical validity, are not as reliable as many scientists assume. Nature 2014, 506, 150–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liakopoulos, V.; Eleftheriadis, T.; Kyropoulos, T.; Voliotis, G.; Potamianos, S.; Zengos, N.; Stefanidis, I.; Heinttz, B. Hemodialysis procedure does not affect the levels of sICAM-1 and sVCAM-1 in patients with end stage renal disease. LRNF 2005, 27, 315–321. [Google Scholar] [CrossRef]
- Muniz-Junqueira, M.I.; Braga Lopes, C.; Magalhães, C.A.M.; Schleicher, C.C.; Veiga, J.P.R. Acute and chronic influence of hemodialysis according to the membrane used on phagocytic function of neutrophils and monocytes and pro-inflammatory cytokines production in chronic renal failure patients. Life Sci. 2005, 77, 3141–3155. [Google Scholar] [CrossRef]
- Tarakçıoğlu, M.; Erbağci, A.B.; Usalan, C.; Deveci, R.; Kocabaş, R. Acute effect of hemodialysis on serum levels of the proinflammatory cytokines. Mediat. Inflamm. 2003, 12, 15–19. [Google Scholar] [CrossRef]
- Szmitko, P.E.; Wang, C.-H.; Weisel, R.D.; de Almeida, J.R.; Anderson, T.J.; Verma, S. New markers of inflammation and endothelial cell activation: Part I. Circulation 2003, 108, 1917–1923. [Google Scholar] [CrossRef]
- Tousoulis, D.; Androulakis, E.; Papageorgiou, N.; Briasoulis, A.; Siasos, G.; Antoniades, C.; Stefanadis, C. From atherosclerosis to acute coronary syndromes: The role of soluble CD40 ligand. Trends Cardiovasc. Med. 2010, 20, 153–164. [Google Scholar] [CrossRef]
- Bu, D.; Griffin, G.; Lichtman, A.H. Mechanisms for the anti-inflammatory effects of statins. Curr. Opin. Lipidol. 2011, 22, 165–170. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulawski, E.; Mellwig, K.-P.; Brinkmann, T.; Kleesiek, K.; Horstkotte, D. Influence of single low-density lipoprotein apheresis on the adhesion molecules soluble vascular cellular adhesion molecule-1, soluble intercellular adhesion molecule-1, and p-selectin. Therapher. Dial. 2002, 6, 229–233. [Google Scholar] [CrossRef]
- Wang, Y.; Blessing, F.; Walli, A.K.; Überfuhr, P.; Fraunberger, P.; Seidel, D. Effects of heparin-mediated extracorporeal low-density lipoprotein precipitation beyond lowering proatherogenic lipoproteins—Reduction of circulating proinflammatory and procoagulatory markers. Atherosclerosis 2004, 175, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, K.; Kawabe, M.; Hirama, A.; Ueda, K.; Kamada, Y.; Arii, K.; Komaba, Y.; Katsura, K.-I.; Lino, Y.; Katayama, Y. Effects of selective LDL apheresis on plasma concentrations of ICAM-1, VCAM-1 and P-selectin in diabetic patients with arteriosclerosis obliterans and receiving maintenance hemodialysis. Clin. Chim. Acta 2007, 377, 198–200. [Google Scholar] [CrossRef]
- Hovland, A.; Hardersen, R.; Sexton, J.; Mollnes, T.E.; LappegÃ¥rd, K.T. Different inflammatory responses induced by three LDL-lowering apheresis columns. J. Clin. Apher. 2009, 24, 247–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef]
- Vaccaro, F.; Mulè, G.; Cottone, S.; Soresi, M.; Giannitrapani, L.; Vadalà, A.; Sparacino, V.; Calabrese, S.; Picone, F.P.; Montalto, G.; et al. Circulating levels of adhesion molecules in chronic kidney disease correlate with the stage of renal disease and with c-reactive protein. Arch. Med. Res. 2007, 38, 534–538. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Heimbürger, O.; Lindholm, B.; Kaysen, G.A.; Bergström, J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol. Dial. Transplant. 2000, 15, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Raines, E.W.; Ferri, N. Cytokines affecting endothelial and smooth muscle cells in vascular disease. Lipid Res. 2005, 46, 1081–1092. [Google Scholar] [CrossRef] [Green Version]
- Koch, A.E.; Halloran, M.M.; Haskell, C.J.; Shah, M.R.; Polverini, P.J. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature 1995, 376, 517–519. [Google Scholar] [CrossRef]
- Lawson, C.; Wolf, S. ICAM-1 signaling in endothelial cells. Pharmacol. Rep. 2009, 61, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular Cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 2011, 15, 1607–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deanfield John, E.; Halcox Julian, P.; Rabelink Ton, J. Endothelial function and dysfunction. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Tripepi, G.; Mallamaci, F.; Zoccali, C. Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: Searching for the best risk marker by multivariate modeling. J. Am. Soc. Nephrol. 2005, 16 (Suppl. 1), S83–S88. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.-F.; Hsu, S.-P.; Pai, M.-F.; Yang, J.-Y.; Chen, H.-Y.; Wu, H.-Y.; Peng, Y.-S. High soluble vascular cell adhesion molecule-1 concentrations predict long-term mortality in hemodialysis patients. Int. Urol. Nephrol. 2013, 45, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Boos, C.J.; Lip, G.Y.H.; Blann, A.D. Circulating endothelial cells in cardiovascular disease. J. Am. Coll. Cardiol. 2006, 48, 1538–1547. [Google Scholar] [CrossRef] [Green Version]
- Chong, A.Y.; Blann, A.D.; Patel, J.; Freestone, B.; Hughes, E.; Lip, G.Y.H. Endothelial dysfunction and damage in congestive heart failure: Relation of flow-mediated dilation to circulating endothelial cells, plasma indexes of endothelial damage, and brain natriuretic peptide. Circulation 2004, 110, 1794–1798. [Google Scholar] [CrossRef] [Green Version]
- Koc, M.; Richards, H.B.; Bihorac, A.; Ross, E.A.; Schold, J.D.; Segal, M.S. Circulating endothelial cells are associated with future vascular events in hemodialysis patients. Kidney Int. 2005, 67, 1078–1083. [Google Scholar] [CrossRef]



| HD Group with Rheopheresis (N = 13) | HD Group without Rheopheresis (N = 10) | |
|---|---|---|
| Age (years old) | 68 (59–71) | 78.5 (73.5–80.5) |
| Male gender | 8 (62%) | 8 (80%) |
| HD Vintage (Months) | 33 (18–39) | 20 (16–35) |
| BMI (kg/m2) | 26.0 (23.2–29.5) | 30.1 (27–71) |
| Diabetes | 8 (62%) | 8 (80%) |
| Hypertension | 13 (100 %) | 8 (80%) |
| Dyslipidemia | 11 (85%) | 6 (60%) |
| Smoking history | 7 (54%) | 7 (60%) |
| Coronary disease | 7 (54%) | 6 (60%) |
| Ejection fraction (%) | 65 (53.7–65) | 50 (50–62.5) |
| Peripheral occlusive arterial disease | 9 (69%) | 9 (90%) |
| Stroke history | 0 | 1 (10%) |
| Arteriovenous fistula | 9 (69%) | 6 (60%) |
| Indication of rheopheresis: | - | |
| PAD-CLTI | 8 | |
| Calciphylaxis | 5 | |
| Number of sessions | 18 (12–30) | - |
| Albumin (g/L) | 31.9 (28.4–35.2) | 35.8 (33.2–39.5) |
| HD Group with Rheopheresis (N = 13) | HD Group without Rheopheresis (N = 10) | p Value | |
|---|---|---|---|
| Fibrinogen (g/L) | 5.9 (5.2–6.5) | 4.4 (4.3–5.6) | 0.11 |
| CRP (mg/L) | 36.0 (12.0–49.0) | 7.9 (6.3–12.3) | 0.01 |
| sICAM-1 (ng/mL) | 243 (54.0–189) | 220 (144–397) | 0.1 |
| sVCAM-1 (ng/mL) | 1735 (1324–2150) | 1146 (862–1442) | 0.04 |
| sE-Selectin (ng/mL) | 25.0 (19.0–35.5) | 34.0 (31.0–49.7) | 0.36 |
| sP-Selectin (ng/mL) | 45.0 (27.5–53.0) | 42.0 (40.0–46.7) | 0.68 |
| sCD40L (pg/mL) | 46.3 (33.9–81.1) | 82.0 (31.0–115) | 0.68 |
| IL-1β (pg/mL) | 1.0 (1.0–1.5) | 1.0 (1.0–4.6) | 0.4 |
| IL-6 (pg/mL) | 6.2 (1.0–17.3) | 1.0 (1.0–1.0) | 0.01 |
| IL-8 (pg/mL) | 11.9 (8.7– 17.7) | 9.0 (6.0–15.5) | 0.23 |
| TNF-α (pg/mL) | 37.8 (29.0–45.1) | 58.0 (50.0–62.0) | 0.0008 |
| IL-10 (pg/mL) | 9.6 (5.1–13.1) | 2.0 (1.0–7.5) | 0.01 |
| Angiopoietin 2 (ng/mL) | 4.1 (3.0–6.9) | 3.0 (3.0–3.9) | 0.18 |
| sEndoglin (pg/mL) | 662 (574–971) | 1347 (912–1455) | 0.01 |
| VEGF-A (pg/mL) | 42.0 (25.0–56.0) | 62.0 (25.0–104) | 0.52 |
| CECs (n/mL) | 16 (2–34) | 1 (1–4) | 0.02 |
| HD Group without Rheopheresis | |||
|---|---|---|---|
| Pre-Dialysis (N = 10) | Post-Dialysis (N = 10) | p Value | |
| ICAM-1 (ng/mL) | 222 (146–347) | 193 (130–317) | 0.04 |
| VCAM-1 (ng/mL) | 1146 (801–1556) | 1120 (701–1629) | 0.49 |
| E-Selectin (ng/mL) | 30.3 (26.6–45.6) | 30.7 (24.5–45.4) | 0.62 |
| P-Selectin (ng/mL) | 41.8 (39.8–48.7) | 48.0 (46.3–64.2) | 0.004 |
| sCD40L (pg/mL) | 81.9 (27.8–179) | 669 (195–881) | 0.002 |
| IL-1β (pg/mL) | 1.6 (1.0–4.6) | 8.7 (0.9–11.6) | 0.027 |
| IL-6 (pg/mL) | 1.0 (1.0–1.0) | 0.9 (0.8–1.5) | 0.57 |
| IL-8 (pg/mL) | 9.3 (5.0–17.4) | 6.9 (4.2–16.6) | 0.08 |
| TNF-α (pg/mL) | 57.8 (49.2–67.3) | 38.8 (31.3–49.8) | 0.009 |
| IL-10 (pg/mL) | 2.2 (1.0–7.5) | 7.0 (1.7–9.8) | 0.19 |
| Angiopoietin (ng/mL) | 3.0 (3.0–4.0) | 2.68 (2.4–3.2) | 0.04 |
| sEndoglin (pg/mL) | 1347 (873–1474) | 1061 (766–1536) | 0.19 |
| VEGF-A (pg/mL) | 59.8 (25.0–113) | 114 (24.0–250) | 0.43 |
| CECs (n/mL) | 1 (1.0–4.0) | 1 (1.0–2.0) | 0.13 |
| HD Group with Rheopheresis | ||||
|---|---|---|---|---|
| Pre-Rheopheresis (N = 37) | Post-Rheopheresis (N = 37) | Percentage Change (%) | p Value | |
| Fibrinogen (g/L) | 4.72 (3.0–6.0) | 1.9(1.4–2.7) | −53.4 [(−59.2)–(−45.4)] | <0.0001 |
| CRP (mg/L) | 15.3(5.2–36.9) | 9.0 (2.8 –21.5) | −39.5 [(−48.1)–(−34.2)] | 0.001 |
| ICAM-1 (ng/mL) | 148 (116–182) | 132 (102–175) | −10 [(−20.7)–(−3.1)] | 0.02 |
| VCAM-1 (ng/mL) | 1856 (1257–2707) | 1330 (908–1654) | −34.7 [(−45.0)–(−23.2)] | <0.0001 |
| E-Selectin (ng/mL) | 21.0 (15.5–30.5) | 12.3 (7.3–20.3) | −38.6 [(−54.8)–(−31.8)] | <0.0001 |
| P-Selectin (ng/mL) | 44.0 (26.5–53.0) | 21.8 (16.1–33.8) | −38.3 [(−48.1)–(−29.1)] | <0.0001 |
| sCD40L (pg/mL) | 44.9 (31.3–64.3) | 61.6 (34.3–96.1) | 38.0 (7.7–104) | 0.06 |
| IL-1β (pg/mL) | 1.0 (1.0–1.0) | 0.9 (0.9–1.07) | −9.1 [(−22.1)–0] | 0.04 |
| IL-6 (pg/mL) | 1.0 (1.0–11.3) | 2.6 (0.9–13.5) | 0 [(−12.4)–37.7] | 0.93 |
| IL-8 (pg/mL) | 10.4 (7.7– 15.6) | 9.6 (7.1–15.1) | −19.4 [(−32.1)–(−6.0)] | 0.99 |
| TNF-α (pg/mL) | 39.1 (30.1–47.0) | 25.7 (22.2–30.4) | −32.6 [(−42.2)–(−22.5)] | <0.0001 |
| IL-10 (pg/mL) | 5.6 (2.1–10.9) | 60.9 (31.3–64.3) | 674 (306–1299) | <0.0001 |
| Angiopoietin2 (ng/mL) | 3.5 (3.0–6.8) | 3.3 (2.8–6.3) | −9.2 [(−14.8)–(−1.6)] | 0.37 |
| sEndoglin (pg/mL) | 694 (353–1018) | 474 (224–697) | −31.5 [(−43.1)–(−17.2)] | <0.0001 |
| VEGF-A (pg/mL) | 25.0 (25.0–56.0) | 26.1 (23.7–46.0) | 0 [(−29.0)–0] | 0.6 |
| CECs (n/mL) | 13 (3–33) | 43 (8–140) | 317 (14.6–574) | 0.002 |
| HD Group with Rheopheresis | |||
|---|---|---|---|
| Pre-Rheopheresis First Session (N = 13) | Pre-Rheopheresis Last Session (N = 13) | p Value | |
| Fibrinogen (g/L) | 5.9 (5.2–6.5) | 3.7 (2.7–4.6) | 0.0007 |
| CRP (mg/L) | 36.0 (12.0–49.0) | 11.5 (2.3–23.1) | 0.12 |
| ICAM-1 (ng/mL) | 243 (54.0–189) | 213 (54.0–159) | 0.04 |
| VCAM-1 (ng/mL) | 1735 (1324–2150) | 2485 (1172–3337) | 0.05 |
| E-Selectin (ng/mL) | 25.0 (19.0–35.5) | 18.0 (14.5–27.5) | 0.009 |
| P-Selectin (ng/mL) | 45.0 (27.5–53.0) | 44.0 (26.0–53.5) | 0.69 |
| sCD40L (pg/mL) | 46.3 (33.9–81.1) | 43.2 (29.9–75.2) | 0.83 |
| IL-1β (pg/mL) | 1.0 (1.0–1.5) | 1.0 (1.0–1.0) | 0.99 |
| IL-6 (pg/mL) | 6.2 (1.0–17.3) | 1.0 (1.0–5.4) | 0.32 |
| IL-8 (pg/mL) | 11.9 (8.7– 17.7) | 10.4 (6.2–14.4) | 0.26 |
| TNF-α (pg/mL) | 37.8 (29.0–45.1) | 40.4 (32.3–50.4) | 0.73 |
| IL-10 (pg/mL) | 9.6 (5.1–13.1) | 3.4 (1.4–8.2) | 0.01 |
| Angiopoietin2 (ng/mL) | 4.1 (3.0–6.9) | 3.0 (3.0–5.8) | 0.71 |
| sEndoglin (pg/mL) | 662 (574–971) | 740 (202.0–1117) | 0.73 |
| VEGF-A (pg/mL) | 42.0 (25.0–56.0) | 30.0 (25.0–53.5) | 0.9 |
| CECs (n/mL) | 16 (2–34) | 10 (2–27) | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solignac, J.; Lacroix, R.; Arnaud, L.; Abdili, E.; Bouchouareb, D.; Burtey, S.; Brunet, P.; Dignat-George, F.; Robert, T. Rheopheresis Performed in Hemodialysis Patients Targets Endothelium and Has an Acute Anti-Inflammatory Effect. J. Clin. Med. 2023, 12, 105. https://doi.org/10.3390/jcm12010105
Solignac J, Lacroix R, Arnaud L, Abdili E, Bouchouareb D, Burtey S, Brunet P, Dignat-George F, Robert T. Rheopheresis Performed in Hemodialysis Patients Targets Endothelium and Has an Acute Anti-Inflammatory Effect. Journal of Clinical Medicine. 2023; 12(1):105. https://doi.org/10.3390/jcm12010105
Chicago/Turabian StyleSolignac, Justine, Romaric Lacroix, Laurent Arnaud, Evelyne Abdili, Dammar Bouchouareb, Stéphane Burtey, Philippe Brunet, Françoise Dignat-George, and Thomas Robert. 2023. "Rheopheresis Performed in Hemodialysis Patients Targets Endothelium and Has an Acute Anti-Inflammatory Effect" Journal of Clinical Medicine 12, no. 1: 105. https://doi.org/10.3390/jcm12010105
APA StyleSolignac, J., Lacroix, R., Arnaud, L., Abdili, E., Bouchouareb, D., Burtey, S., Brunet, P., Dignat-George, F., & Robert, T. (2023). Rheopheresis Performed in Hemodialysis Patients Targets Endothelium and Has an Acute Anti-Inflammatory Effect. Journal of Clinical Medicine, 12(1), 105. https://doi.org/10.3390/jcm12010105