Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA): Focus on Coronary Microvascular Dysfunction and Genetic Susceptibility
Abstract
:1. Introduction
2. Pathophysiology and Underlying Mechanisms of MINOCA
2.1. Coronary Physiology and Regulation of Coronary Blood Flow
2.2. MINOCA and Pathophysiological Mechanisms
3. Coronary Microvascular Dysfunction in MINOCA
4. Invasive and Non-Invasive Assessment of Coronary Microvascular Dysfunction
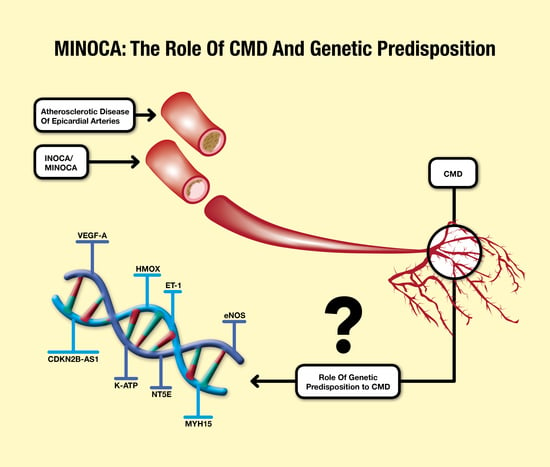
5. MINOCA and Coronary Microvascular Dysfunction: The Genetic Susceptibility
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- DeWood, M.A.; Spores, J.; Notske, R.; Mouser, L.T.; Burroughs, R.; Golden, M.S.; Lang, H.T. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N. Engl. J. Med. 1980, 303, 897–902. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2017, 38, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, M.; Ashokprabhu, N.; Shewale, A.; Pico, M.; Henry, T.D.; Quesada, O. Myocardial infarction with non-obstructive coronary arteries (MINOCA). Front. Cardiovasc. Med. 2022, 9, 1032436. [Google Scholar] [CrossRef] [PubMed]
- Tamis-Holland, J.E.; Jneid, H.; Reynolds, H.R.; Agewall, S.; Brilakis, E.S.; Brown, T.M.; Lerman, A.; Cushman, M.; Kumbhani, D.J.; Arslanian-Engoren, C.; et al. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e891–e908. [Google Scholar] [CrossRef] [PubMed]
- Bainey, K.R.; Welsh, R.C.; Alemayehu, W.; Westerhout, C.M.; Traboulsi, D.; Anderson, T.; Brass, N.; Armstrong, P.W.; Kaul, P. Population-level incidence and outcomes of myocardial infarction with non-obstructive coronary arteries (MINOCA): Insights from the Alberta contemporary acute coronary syndrome patients invasive treatment strategies (COAPT) study. Int. J. Cardiol. 2018, 264, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Pilote, L.; Karp, I. GENESIS-PRAXY (GENdEr and Sex determInantS of cardiovascular disease: From bench to beyond-Premature Acute Coronary SYndrome). Am. Heart J. 2012, 163, 741–746.e2. [Google Scholar] [CrossRef]
- Hansen, B.; Holtzman, J.N.; Juszczynski, C.; Khan, N.; Kaur, G.; Varma, B.; Gulati, M. Ischemia with No Obstructive Arteries (INOCA): A Review of the Prevalence, Diagnosis and Management. Curr. Probl. Cardiol. 2023, 48, 101420. [Google Scholar] [CrossRef]
- Antony, I.; Nitenberg, A.; Foult, J.M.; Aptecar, E. Coronary vasodilator reserve in untreated and treated hypertensive patients with and without left ventricular hypertrophy. J. Am. Coll. Cardiol. 1993, 22, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.A.; Gnecchi-Ruscone, T.; Schäfers, K.P.; Lüscher, T.F.; Camici, P.G. Low density lipoprotein cholesterol and coronary microvascular dysfunction in hypercholesterolemia. J. Am. Coll. Cardiol. 2000, 36, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Szolc, P.; Niewiara, Ł.; Kleczyński, P.; Bryniarski, K.; Ostrowska-Kaim, E.; Szkodoń, K.; Brzychczy, P.; Żmudka, K.; Legutko, J.; Guzik, B. Clinical Characteristics Predicting Worse Long-Term Outcomes in Patients with Myocardial Infarction and Non-Obstructive Coronary Arteries (MINOCA). J. Cardiovasc. Dev. Dis. 2022, 9, 286. [Google Scholar] [CrossRef] [PubMed]
- Di Carli, M.F.; Janisse, J.; Grunberger, G.; Ager, J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J. Am. Coll. Cardiol. 2003, 41, 1387–1393. [Google Scholar] [CrossRef]
- Cosín-Sales, J.; Pizzi, C.; Brown, S.; Kaski, J.C. C-reactive protein, clinical presentation, and ischemic activity in patients with chest pain and normal coronary angiograms. J. Am. Coll. Cardiol. 2003, 41, 1468–1474. [Google Scholar] [CrossRef]
- Safdar, B.; Spatz, E.S.; Dreyer, R.P.; Beltrame, J.F.; Lichtman, J.H.; Spertus, J.A.; Reynolds, H.R.; Geda, M.; Bueno, H.; Dziura, J.D.; et al. Presentation, Clinical Profile, and Prognosis of Young Patients With Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): Results From the VIRGO Study. J. Am. Heart Assoc. 2018, 7, e009174. [Google Scholar] [CrossRef]
- Taqueti, V.R.; Di Carli, M.F. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2625–2641. [Google Scholar] [CrossRef]
- Baumgart, D.; Naber, C.; Haude, M.; Oldenburg, O.; Erbel, R.; Heusch, G.; Siffert, W. G protein beta3 subunit 825T allele and enhanced coronary vasoconstriction on alpha(2)-adrenoceptor activation. Circ. Res. 1999, 85, 965–969. [Google Scholar] [CrossRef]
- Tune, J.D. Withdrawal of vasoconstrictor influences in local metabolic coronary vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2044–H2046. [Google Scholar] [CrossRef]
- Magenta, A.; Greco, S.; Capogrossi, M.C.; Gaetano, C.; Martelli, F. Nitric oxide, oxidative stress, and p66Shc interplay in diabetic endothelial dysfunction. BioMed Res. Int. 2014, 2014, 193095. [Google Scholar] [CrossRef]
- Tsai, S.H.; Lu, G.; Xu, X.; Ren, Y.; Hein, T.W.; Kuo, L. Enhanced endothelin-1/Rho-kinase signalling and coronary microvascular dysfunction in hypertensive myocardial hypertrophy. Cardiovasc. Res. 2017, 113, 1329–1337. [Google Scholar] [CrossRef]
- Chilian, W.M.; Harrison, D.G.; Haws, C.W.; Snyder, W.D.; Marcus, M.L. Adrenergic coronary tone during submaximal exercise in the dog is produced by circulating catecholamines. Evidence for adrenergic denervation supersensitivity in the myocardium but not in coronary vessels. Circ. Res. 1986, 58, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Chilian, W.M.; Ackell, P.H. Transmural differences in sympathetic coronary constriction during exercise in the presence of coronary stenosis. Circ. Res. 1988, 62, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Goodwill, A.G.; Dick, G.M.; Kiel, A.M.; Tune, J.D. Regulation of Coronary Blood Flow. Compr. Physiol. 2017, 7, 321–382. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, N.; Boer, C.; Lamberts, R.R.; Sipkema, P. Cross-talk between cardiac muscle and coronary vasculature. Physiol. Rev. 2006, 86, 1263–1308. [Google Scholar] [CrossRef]
- Dick, G.M.; Tune, J.D. Role of potassium channels in coronary vasodilation. Exp. Biol. Med. 2010, 235, 10–22. [Google Scholar] [CrossRef]
- Fedele, F.; Mancone, M.; Chilian, W.M.; Severino, P.; Canali, E.; Logan, S.; De Marchis, M.L.; Volterrani, M.; Palmirotta, R.; Guadagni, F. Role of genetic polymorphisms of ion channels in the pathophysiology of coronary microvascular dysfunction and ischemic heart disease. Basic Res. Cardiol. 2013, 108, 387. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Birtolo, L.I.; Mariani, M.V.; Lavalle, C.; Maestrini, V.; Mancone, M.; Fedele, F. Ischemic Heart Disease and Heart Failure: Role of Coronary Ion Channels. Int. J. Mol. Sci. 2020, 21, 3167. [Google Scholar] [CrossRef]
- Scalone, G.; Niccoli, G.; Crea, F. Pathophysiology, diagnosis and management of MINOCA: An update. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 54–62. [Google Scholar] [CrossRef]
- Tweet, M.S.; Hayes, S.N.; Pitta, S.R.; Simari, R.D.; Lerman, A.; Lennon, R.J.; Gersh, B.J.; Khambatta, S.; Best, P.J.; Rihal, C.S.; et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012, 126, 579–588. [Google Scholar] [CrossRef]
- Khan, A.; Lahmar, A.; Riasat, M.; Ehtesham, M.; Asif, H.; Khan, W.; Haseeb, M.; Boricha, H. Myocardial Infarction With Non-obstructive Coronary Arteries: An Updated Overview of Pathophysiology, Diagnosis, and Management. Cureus 2022, 14, e23602. [Google Scholar] [CrossRef]
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am. Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, F.; Kaski, J.C.; Crea, F.; Camici, P.G. Pathophysiology of Takotsubo Syndrome. Circulation 2017, 135, 2426–2441. [Google Scholar] [CrossRef]
- Lopez-Pais, J.; Izquierdo Coronel, B.; Raposeiras-Roubín, S.; Álvarez Rodriguez, L.; Vedia, O.; Almendro-Delia, M.; Sionis, A.; Martin-Garcia, A.C.; Uribarri, A.; Blanco, E.; et al. Differences Between Takotsubo and the Working Diagnosis of Myocardial Infarction With Nonobstructive Coronary Arteries. Front. Cardiovasc. Med. 2022, 9, 742010. [Google Scholar] [CrossRef] [PubMed]
- Stepien, K.; Nowak, K.; Szlosarczyk, B.; Nessler, J.; Zalewski, J. Clinical Characteristics and Long-Term Outcomes of MINOCA Accompanied by Active Cancer: A Retrospective Insight Into a Cardio-Oncology Center Registry. Front. Cardiovasc. Med. 2022, 9, 785246. [Google Scholar] [CrossRef]
- Crea, F.; Camici, P.G.; Bairey Merz, C.N. Coronary microvascular dysfunction: An update. Eur. Heart J. 2014, 35, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; d’Amati, G.; Rimoldi, O. Coronary microvascular dysfunction: Mechanisms and functional assessment. Nat. Rev. Cardiol. 2015, 12, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, T.; Merinopoulos, I.; Wickramarachchi, U.; Vassiliou, V.; Eccleshall, S. Endothelial Dysfunction and Coronary Vasoreactivity—A Review of the History, Physiology, Diagnostic Techniques, and Clinical Relevance. Curr. Cardiol. Rev. 2021, 17, 85–100. [Google Scholar] [CrossRef]
- Brutsaert, D.L. Cardiac endothelial-myocardial signaling: Its role in cardiac growth, contractile performance, and rhythmicity. Physiol. Rev. 2003, 83, 59–115. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Ong, P.; Sechtem, U.; Beltrame, J.; Camici, P.G.; Crea, F.; Kaski, J.C.; Bairey Merz, C.N.; Pepine, C.J.; Shimokawa, H.; et al. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease: Why, How, and When. JACC Cardiovasc. Interv. 2020, 13, 1847–1864. [Google Scholar] [CrossRef]
- Mileva, N.; Nagumo, S.; Mizukami, T.; Sonck, J.; Berry, C.; Gallinoro, E.; Monizzi, G.; Candreva, A.; Munhoz, D.; Vassilev, D.; et al. Prevalence of Coronary Microvascular Disease and Coronary Vasospasm in Patients With Nonobstructive Coronary Artery Disease: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2022, 11, e023207. [Google Scholar] [CrossRef]
- Abdu, F.A.; Liu, L.; Mohammed, A.Q.; Yin, G.; Xu, B.; Zhang, W.; Xu, S.; Lv, X.; Fan, R.; Feng, C.; et al. Prognostic impact of coronary microvascular dysfunction in patients with myocardial infarction with non-obstructive coronary arteries. Eur. J. Intern. Med. 2021, 92, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Shin, D.; Lee, J.M.; van de Hoef, T.P.; Hong, D.; Choi, K.H.; Hwang, D.; Boerhout, C.K.M.; de Waard, G.A.; Jung, J.H.; et al. Clinical Relevance of Ischemia with Nonobstructive Coronary Arteries According to Coronary Microvascular Dysfunction. J. Am. Heart Assoc. 2022, 11, e025171. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Takeyama, Y.; Koba, S.; Suwa, Y.; Katagiri, T. Small vessel pathology and coronary hemodynamics in patients with microvascular angina. Int. J. Cardiol. 1994, 43, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.; Lindner, D.; Kasner, M.; Zietsch, C.; Savvatis, K.; Escher, F.; von Schlippenbach, J.; Skurk, C.; Steendijk, P.; Riad, A.; et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ. Heart Fail. 2011, 4, 44–52. [Google Scholar] [CrossRef]
- Goligorsky, M.S. Microvascular rarefaction: The decline and fall of blood vessels. Organogenesis 2010, 6, 1–10. [Google Scholar] [CrossRef]
- Mohri, M.; Koyanagi, M.; Egashira, K.; Tagawa, H.; Ichiki, T.; Shimokawa, H.; Takeshita, A. Angina pectoris caused by coronary microvascular spasm. Lancet 1998, 351, 1165–1169. [Google Scholar] [CrossRef]
- Heusch, G.; Skyschally, A.; Kleinbongard, P. Coronary microembolization and microvascular dysfunction. Int. J. Cardiol. 2018, 258, 17–23. [Google Scholar] [CrossRef]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef]
- Vancheri, F.; Longo, G.; Vancheri, S.; Henein, M. Coronary Microvascular Dysfunction. J. Clin. Med. 2020, 9, 2880. [Google Scholar] [CrossRef]
- Montone, R.A.; Niccoli, G.; Fracassi, F.; Russo, M.; Gurgoglione, F.; Cammà, G.; Lanza, G.A.; Crea, F. Patients with acute myocardial infarction and non-obstructive coronary arteries: Safety and prognostic relevance of invasive coronary provocative tests. Eur. Heart J. 2018, 39, 91–98. [Google Scholar] [CrossRef]
- Kinlay, S.; Libby, P.; Ganz, P. Endothelial function and coronary artery disease. Curr. Opin. Lipidol. 2001, 12, 383–389. [Google Scholar] [CrossRef]
- Stępień, K.; Nowak, K.; Nessler, J.; Zalewski, J. Worse long-term prognosis in myocardial infarction occurring at weekends or public holidays with insight into myocardial infarction with nonobstructive coronary arteries. Pol. Arch. Intern. Med. 2020, 130, 942–952. [Google Scholar] [CrossRef]
- Stepien, K.; Nowak, K.; Wypasek, E.; Zalewski, J.; Undas, A. High prevalence of inherited thrombophilia and antiphospholipid syndrome in myocardial infarction with non-obstructive coronary arteries: Comparison with cryptogenic stroke. Int. J. Cardiol. 2019, 290, 1–6. [Google Scholar] [CrossRef]
- Popovic, B.; Agrinier, N.; Bouchahda, N.; Pinelli, S.; Maigrat, C.H.; Metzdorf, P.A.; Selton Suty, C.; Juillière, Y.; Camenzind, E. Coronary Embolism Among ST-Segment-Elevation Myocardial Infarction Patients: Mechanisms and Management. Circ. Cardiovasc. Interv. 2018, 11, e005587. [Google Scholar] [CrossRef] [PubMed]
- Tornvall, P.; Gerbaud, E.; Behaghel, A.; Chopard, R.; Collste, O.; Laraudogoitia, E.; Leurent, G.; Meneveau, N.; Montaudon, M.; Perez-David, E.; et al. Myocarditis or “true” infarction by cardiac magnetic resonance in patients with a clinical diagnosis of myocardial infarction without obstructive coronary disease: A meta-analysis of individual patient data. Atherosclerosis 2015, 241, 87–91. [Google Scholar] [CrossRef]
- Mahrholdt, H.; Wagner, A.; Deluigi, C.C.; Kispert, E.; Hager, S.; Meinhardt, G.; Vogelsberg, H.; Fritz, P.; Dippon, J.; Bock, C.T.; et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006, 114, 1581–1590. [Google Scholar] [CrossRef]
- Ong, P.; Athanasiadis, A.; Borgulya, G.; Mahrholdt, H.; Kaski, J.C.; Sechtem, U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). J. Am. Coll. Cardiol. 2012, 59, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Amiya, E.; Watanabe, M.; Komuro, I. The Relationship between Vascular Function and the Autonomic Nervous System. Ann. Vasc. Dis. 2014, 7, 109–119. [Google Scholar] [CrossRef]
- Koller, A.; Laughlin, M.H.; Cenko, E.; de Wit, C.; Tóth, K.; Bugiardini, R.; Trifunovits, D.; Vavlukis, M.; Manfrini, O.; Lelbach, A.; et al. Functional and structural adaptations of the coronary macro- and microvasculature to regular aerobic exercise by activation of physiological, cellular, and molecular mechanisms: ESC Working Group on Coronary Pathophysiology and Microcirculation position paper. Cardiovasc. Res. 2022, 118, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Bland, A.; Chuah, E.; Meere, W.; Ford, T.J. Targeted Therapies for Microvascular Disease. Interv. Cardiol. Clin. 2023, 12, 131–139. [Google Scholar] [CrossRef]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef]
- Van’t Veer, M.; Pijls, N.H.J.; Hennigan, B.; Watkins, S.; Ali, Z.A.; De Bruyne, B.; Zimmermann, F.M.; van Nunen, L.X.; Barbato, E.; Berry, C.; et al. Comparison of Different Diastolic Resting Indexes to iFR: Are They All Equal? J. Am. Coll. Cardiol. 2017, 70, 3088–3096. [Google Scholar] [CrossRef]
- Candreva, A.; Gallinoro, E.; van ‘t Veer, M.; Sonck, J.; Collet, C.; Di Gioia, G.; Kodeboina, M.; Mizukami, T.; Nagumo, S.; Keulards, D.; et al. Basics of Coronary Thermodilution. JACC Cardiovasc. Interv. 2021, 14, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Fahrni, G.; Wolfrum, M.; De Maria, G.L.; Cuculi, F.; Dawkins, S.; Alkhalil, M.; Patel, N.; Forfar, J.C.; Prendergast, B.D.; Choudhury, R.P.; et al. Index of Microcirculatory Resistance at the Time of Primary Percutaneous Coronary Intervention Predicts Early Cardiac Complications: Insights From the OxAMI (Oxford Study in Acute Myocardial Infarction) Cohort. J. Am. Heart Assoc. 2017, 6, e005409. [Google Scholar] [CrossRef] [PubMed]
- De Maria, G.L.; Alkhalil, M.; Wolfrum, M.; Fahrni, G.; Borlotti, A.; Gaughran, L.; Dawkins, S.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; et al. Index of Microcirculatory Resistance as a Tool to Characterize Microvascular Obstruction and to Predict Infarct Size Regression in Patients With STEMI Undergoing Primary PCI. JACC Cardiovasc. Imaging 2019, 12, 837–848. [Google Scholar] [CrossRef]
- Kang, M.G.; Koo, B.K.; Tantry, U.S.; Kim, K.; Ahn, J.H.; Park, H.W.; Park, J.R.; Hwang, S.J.; Hwang, J.Y.; Gurbel, P.A.; et al. Association Between Thrombogenicity Indices and Coronary Microvascular Dysfunction in Patients With Acute Myocardial Infarction. Basic Transl. Sci. 2021, 6, 749–761. [Google Scholar] [CrossRef]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J. Am. Coll. Cardiol. 2018, 72 Pt A, 2841–2855. [Google Scholar] [CrossRef]
- Murai, T.; Yonetsu, T.; Kanaji, Y.; Usui, E.; Hoshino, M.; Hada, M.; Hamaya, R.; Kanno, Y.; Lee, T.; Kakuta, T. Prognostic value of the index of microcirculatory resistance after percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndrome. Catheter. Cardiovasc. Interv. 2018, 92, 1063–1074. [Google Scholar] [CrossRef]
- De Maria, G.L.; Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.; et al. Angiography-derived index of microcirculatory resistance as a novel, pressure-wire-free tool to assess coronary microcirculation in ST elevation myocardial infarction. Int. J. Cardiovasc. Imaging 2020, 36, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; OxAMI Study Investigators; Ribichini, F.; Ferreira, V.M.; et al. Angiography-derived index of microcirculatory resistance (IMRangio) as a novel pressure-wire-free tool to assess coronary microvascular dysfunction in acute coronary syndromes and stable coronary artery disease. Int. J. Cardiovasc. Imaging 2021, 37, 1801–1813. [Google Scholar] [CrossRef]
- Choi, K.H.; Dai, N.; Li, Y.; Kim, J.; Shin, D.; Lee, S.H.; Joh, H.S.; Kim, H.K.; Jeon, K.H.; Ha, S.J.; et al. Functional Coronary Angiography-Derived Index of Microcirculatory Resistance in Patients With ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2021, 14, 1670–1684. [Google Scholar] [CrossRef] [PubMed]
- Everaars, H.; de Waard, G.A.; Driessen, R.S.; Danad, I.; van de Ven, P.M.; Raijmakers, P.G.; Lammertsma, A.A.; van Rossum, A.C.; Knaapen, P.; van Royen, N. Doppler Flow Velocity and Thermodilution to Assess Coronary Flow Reserve: A Head-to-Head Comparison With [15O]H2O PET. JACC Cardiovasc. Interv. 2018, 11, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
- Pijls, N.H.; De Bruyne, B.; Smith, L.; Aarnoudse, W.; Barbato, E.; Bartunek, J.; Bech, G.J.; Van De Vosse, F. Coronary thermodilution to assess flow reserve: Validation in humans. Circulation 2002, 105, 2482–2486. [Google Scholar] [CrossRef]
- Fearon, W.F.; Balsam, L.B.; Farouque, H.M.; Caffarelli, A.D.; Robbins, R.C.; Fitzgerald, P.J.; Yock, P.G.; Yeung, A.C. Novel index for invasively assessing the coronary microcirculation. Circulation 2003, 107, 3129–3132. [Google Scholar] [CrossRef]
- Jansen, T.P.J.; Konst, R.E.; Elias-Smale, S.E.; van den Oord, S.C.; Ong, P.; de Vos, A.M.J.; van de Hoef, T.P.; Paradies, V.; Smits, P.C.; van Royen, N.; et al. Assessing Microvascular Dysfunction in Angina With Unobstructed Coronary Arteries: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 78, 1471–1479. [Google Scholar] [CrossRef]
- Feher, A.; Sinusas, A.J. Quantitative Assessment of Coronary Microvascular Function: Dynamic Single-Photon Emission Computed Tomography, Positron Emission Tomography, Ultrasound, Computed Tomography, and Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2017, 10, e006427. [Google Scholar] [CrossRef]
- Ayub, M.T.; Kalra, D. Coronary Microvascular Dysfunction and the Role of Noninvasive Cardiovascular Imaging. Diagnostics 2020, 10, 679. [Google Scholar] [CrossRef]
- Vegsundvåg, J.; Holte, E.; Wiseth, R.; Hegbom, K.; Hole, T. Coronary flow velocity reserve in the three main coronary arteries assessed with transthoracic Doppler: A comparative study with quantitative coronary angiography. J. Am. Soc. Echocardiogr. 2011, 24, 758–767. [Google Scholar] [CrossRef]
- Schroder, J.; Michelsen, M.M.; Mygind, N.D.; Suhrs, H.E.; Bove, K.B.; Bechsgaard, D.F.; Aziz, A.; Gustafsson, I.; Kastrup, J.; Prescott, E. Coronary flow velocity reserve predicts adverse prognosis in women with angina and no obstructive coronary artery disease: Results from the iPOWER study. Eur. Heart J. 2021, 42, 228–239. [Google Scholar] [CrossRef]
- Thomson, L.E.; Wei, J.; Agarwal, M.; Haft-Baradaran, A.; Shufelt, C.; Mehta, P.K.; Gill, E.B.; Johnson, B.D.; Kenkre, T.; Handberg, E.M.; et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ. Cardiovasc. Imaging 2015, 8, e002481. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, T.A.; Jerosch-Herold, M.; Misselwitz, B.; Zhang, H.; Gropler, R.J.; Zheng, J. Fast mapping of myocardial blood flow with MR first-pass perfusion imaging. Magn. Reson. Med. 2008, 59, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Indorkar, R.; Kwong, R.Y.; Romano, S.; White, B.E.; Chia, R.C.; Trybula, M.; Evans, K.; Shenoy, C.; Farzaneh-Far, A. Global Coronary Flow Reserve Measured During Stress Cardiac Magnetic Resonance Imaging Is an Independent Predictor of Adverse Cardiovascular Events. JACC Cardiovasc. Imaging 2019, 12 Pt 2, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R. Chromosome 9p21 and coronary artery disease. N. Engl. J. Med. 2010, 362, 1736–1737. [Google Scholar] [CrossRef]
- Kessler, T.; Vilne, B.; Schunkert, H. The impact of genome-wide association studies on the pathophysiology and therapy of cardiovascular disease. EMBO Mol. Med. 2016, 8, 688–701. [Google Scholar] [CrossRef]
- Yoshino, S.; Cilluffo, R.; Best, P.J.; Atkinson, E.J.; Aoki, T.; Cunningham, J.M.; de Andrade, M.; Choi, B.J.; Lerman, L.O.; Lerman, A. Single nucleotide polymorphisms associated with abnormal coronary microvascular function. Coron. Artery Dis. 2014, 25, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Helgadottir, A.; Thorleifsson, G.; Manolescu, A.; Gretarsdottir, S.; Blondal, T.; Jonasdottir, A.; Jonasdottir, A.; Sigurdsson, A.; Baker, A.; Palsson, A.; et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science 2007, 316, 1491–1493. [Google Scholar] [CrossRef]
- Harismendy, O.; Notani, D.; Song, X.; Rahim, N.G.; Tanasa, B.; Heintzman, N.; Ren, B.; Fu, X.D.; Topol, E.J.; Rosenfeld, M.G.; et al. 9p21 DNA variants associated with coronary artery disease impair interferon-γ signalling response. Nature 2011, 470, 264–268. [Google Scholar] [CrossRef]
- Simón-Yarza, T.; Formiga, F.R.; Tamayo, E.; Pelacho, B.; Prosper, F.; Blanco-Prieto, M.J. Vascular endothelial growth factor-delivery systems for cardiac repair: An overview. Theranostics 2012, 2, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.W.; Lee, W.J.; Lee, I.T.; Lee, W.L.; Wang, J.S.; Wu, J.P.; Sheu, W.H. Subjects with microvascular angina have longer GT repeats polymorphism in the haem oxygenase-1 gene promoter. Biomarkers 2020, 25, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.L.; Kong, S.M.Y.; Tumanov, S.; Chen, W.; Cantley, J.; Ayer, A.; Maghzal, G.J.; Midwinter, R.G.; Chan, K.H.; Ng, M.K.C.; et al. Hmox1 (Heme Oxygenase-1) Protects Against Ischemia-Mediated Injury via Stabilization of HIF-1α (Hypoxia-Inducible Factor-1α). Arterioscler. Thromb. Vasc. Biol. 2021, 41, 317–330. [Google Scholar] [CrossRef]
- Halcox, J.P.J.; Nour, K.R.A.; Zalos, G.; Quyyumi, A.A. Endogenous endothelin in human coronary vascular function: Differential contribution of endothelin receptor types A and B. Hypertension 2007, 49, 1134–1141. [Google Scholar] [CrossRef]
- Gupta, R.M.; Hadaya, J.; Trehan, A.; Zekavat, S.M.; Roselli, C.; Klarin, D.; Emdin, C.A.; Hilvering, C.R.E.; Bianchi, V.; Mueller, C.; et al. A Genetic Variant Associated with Five Vascular Diseases Is a Distal Regulator of Endothelin-1 Gene Expression. Cell 2017, 170, 522–533.e15. [Google Scholar] [CrossRef] [PubMed]
- Cox, I.D.; Bøtker, H.E.; Bagger, J.P.; Sonne, H.S.; Kristensen, B.O.; Kaski, J.C. Elevated endothelin concentrations are associated with reduced coronary vasomotor responses in patients with chest pain and normal coronary arteriograms. J. Am. Coll. Cardiol. 1999, 34, 455–460. [Google Scholar] [CrossRef]
- Ford, T.J.; Corcoran, D.; Padmanabhan, S.; Aman, A.; Rocchiccioli, P.; Good, R.; McEntegart, M.; Maguire, J.J.; Watkins, S.; Eteiba, H.; et al. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. Eur. Heart J. 2020, 41, 3239–3252. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Prosperi, S.; Magnocavallo, M.; Mariani, M.V.; Netti, L.; Birtolo, L.I.; De Orchi, P.; Chimenti, C.; Maestrini, V.; et al. Potential Role of eNOS Genetic Variants in Ischemic Heart Disease Susceptibility and Clinical Presentation. J. Cardiovasc. Dev. Dis. 2021, 8, 116. [Google Scholar] [CrossRef]
- Fedele, F.; Severino, P.; Bruno, N.; Stio, R.; Caira, C.; D’Ambrosi, A.; Brasolin, B.; Ohanyan, V.; Mancone, M. Role of ion channels in coronary microcirculation: A review of the literature. Future Cardiol. 2013, 9, 897–905. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Netti, L.; Pucci, M.; Mariani, M.V.; Cimino, S.; Birtolo, L.I.; Infusino, F.; De Orchi, P.; Palmirotta, R.; et al. Susceptibility to ischaemic heart disease: Focusing on genetic variants for ATP-sensitive potassium channel beyond traditional risk factors. Eur. J. Prev. Cardiol. 2021, 28, 1495–1500. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Mancone, M.; Palazzuoli, A.; Mariani, M.V.; Prosperi, S.; Myftari, V.; Lavalle, C.; Forleo, G.B.; Birtolo, L.I.; et al. Protection against Ischemic Heart Disease: A Joint Role for eNOS and the KATP Channel. Int. J. Mol. Sci. 2023, 24, 7927. [Google Scholar] [CrossRef] [PubMed]
- Ohanyan, V.; Yin, L.; Bardakjian, R.; Kolz, C.; Enrick, M.; Hakobyan, T.; Luli, J.; Graham, K.; Khayata, M.; Logan, S.; et al. Kv1.3 channels facilitate the connection between metabolism and blood flow in the heart. Microcirculation 2017, 24, e12334. [Google Scholar] [CrossRef] [PubMed]
- Ohanyan, V.; Yin, L.; Bardakjian, R.; Kolz, C.; Enrick, M.; Hakobyan, T.; Kmetz, J.; Bratz, I.; Luli, J.; Nagane, M.; et al. Requisite Role of Kv1.5 Channels in Coronary Metabolic Dilation. Circ. Res. 2015, 117, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Maffei, S.; Guiducci, L.; Cugusi, L.; Cadeddu, C.; Deidda, M.; Gallina, S.; Sciomer, S.; Gastaldelli, A.; Kaski, J.C.; Working Group on “Gender difference in cardiovascular disease” of the Italian Society of Cardiology. Women-specific predictors of cardiovascular disease risk—New paradigms. Int. J. Cardiol. 2019, 286, 190–197. [Google Scholar] [CrossRef]
- Vaccarino, V.; Badimon, L.; Corti, R.; de Wit, C.; Dorobantu, M.; Hall, A.; Koller, A.; Marzilli, M.; Pries, A.; Bugiardini, R.; et al. Ischaemic heart disease in women: Are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc. Res. 2011, 90, 9–17. [Google Scholar] [CrossRef]
- Pacheco Claudio, C.; Quesada, O.; Pepine, C.J.; Noel Bairey Merz, C. Why names matter for women: MINOCA/INOCA (myocardial infarction/ischemia and no obstructive coronary artery disease). Clin. Cardiol. 2018, 41, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Pepine, C.J.; Anderson, R.D.; Sharaf, B.L.; Reis, S.E.; Smith, K.M.; Handberg, E.M.; Johnson, B.D.; Sopko, G.; Bairey Merz, C.N. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J. Am. Coll. Cardiol. 2010, 55, 2825–2832. [Google Scholar] [CrossRef] [PubMed]
- St Hilaire, C.; Ziegler, S.G.; Markello, T.C.; Brusco, A.; Groden, C.; Gill, F.; Carlson-Donohoe, H.; Lederman, R.J.; Chen, M.Y.; Yang, D.; et al. NT5E mutations and arterial calcifications. N. Engl. J. Med. 2011, 364, 432–442. [Google Scholar] [CrossRef]
- Pósfai, É.; Marton, I.; Borbényi, Z.; Nemes, A. Myocardial infarction as a thrombotic complication of essential thrombocythemia and polycythemia vera. Anatol. J. Cardiol. 2016, 16, 397–402. [Google Scholar] [CrossRef]
- Cella, G.; Marchetti, M.; Vianello, F.; Panova-Noeva, M.; Vignoli, A.; Russo, L.; Barbui, T.; Falanga, A. Nitric oxide derivatives and soluble plasma selectins in patients with myeloproliferative neoplasms. Thromb. Haemost. 2010, 104, 151–156. [Google Scholar] [CrossRef]
- Aoyama, R.; Kubota, Y.; Tara, S.; Wakita, S.; Yamaguchi, H.; Shimizu, W.; Takano, H. Vascular Endothelial Dysfunction in Myeloproliferative Neoplasms and Gene Mutations. Int. Heart J. 2022, 63, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Ramaraj, R.; Movahed, M.R. Microvascular dysfunction following primary percutaneous coronary intervention in the setting of ST-elevation myocardial infarction. J. Invasive Cardiol. 2008, 20, 603–614. [Google Scholar] [PubMed]
- Cui, L.; Han, L.; Wang, J.; Huang, P.; Tian, G.; Wang, Y.; Li, J. Prevalence and characteristics of coronary microvascular dysfunction in post-percutaneous coronary intervention patients with recurrent chest pain. Cardiovasc. Diagn. Ther. 2022, 12, 166–176. [Google Scholar] [CrossRef] [PubMed]


| Protein | Pathophysiological Mechanism | Reference |
|---|---|---|
| VEGF-A | Reduced expression → Repairing mechanisms abnormalities and increased apoptotic process → VSMCs and endothelial cells dysfunction → CMD | [88] |
| CDKN2B-AS1 | Deficiency → abnormalities in VSMCs and endothelial cells proliferation and senescence → CMD | [88,89] |
| HMOX | SNPs → reduced protection against ischemic injury → CMD | [92,93] |
| ET-1 | SNP rs9349379-G → increased plasma concentration of ET-1 → vasomotor tone impairment and atherosclerotic disease progression → CAD and CMD | [94,95,96,97] |
| eNOS | SNP rs1799983_G/T → substitution of guanine with thymine with consequent aminoacidic change from glutamic acid to aspartic acid → lower mRNA levels → reduction in eNOS expression → endothelial dysfunction → CAD and CMD | [98,99] |
| K-ATP | SNP rs5215_G/G of KCNJ11 → valine–isoleucine substitution → K-ATP gain of function → increased vasodilation and shear stress reduction. | [100,101] |
| NT5E | Genetic variants → CFR reduction and increased coronary calcification | [88,108] |
| MYH15 | Deregulation of vascular tone → increased risk of CMD | [88] |
| JAK2 | V617F mutation → endothelial dysfunction and coronary spasm | [109,110,111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Severino, P.; D’Amato, A.; Prosperi, S.; Myftari, V.; Colombo, L.; Tomarelli, E.; Piccialuti, A.; Di Pietro, G.; Birtolo, L.I.; Maestrini, V.; et al. Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA): Focus on Coronary Microvascular Dysfunction and Genetic Susceptibility. J. Clin. Med. 2023, 12, 3586. https://doi.org/10.3390/jcm12103586
Severino P, D’Amato A, Prosperi S, Myftari V, Colombo L, Tomarelli E, Piccialuti A, Di Pietro G, Birtolo LI, Maestrini V, et al. Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA): Focus on Coronary Microvascular Dysfunction and Genetic Susceptibility. Journal of Clinical Medicine. 2023; 12(10):3586. https://doi.org/10.3390/jcm12103586
Chicago/Turabian StyleSeverino, Paolo, Andrea D’Amato, Silvia Prosperi, Vincenzo Myftari, Lorenzo Colombo, Elisa Tomarelli, Alice Piccialuti, Gianluca Di Pietro, Lucia Ilaria Birtolo, Viviana Maestrini, and et al. 2023. "Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA): Focus on Coronary Microvascular Dysfunction and Genetic Susceptibility" Journal of Clinical Medicine 12, no. 10: 3586. https://doi.org/10.3390/jcm12103586
APA StyleSeverino, P., D’Amato, A., Prosperi, S., Myftari, V., Colombo, L., Tomarelli, E., Piccialuti, A., Di Pietro, G., Birtolo, L. I., Maestrini, V., Badagliacca, R., Sardella, G., Fedele, F., Vizza, C. D., & Mancone, M. (2023). Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA): Focus on Coronary Microvascular Dysfunction and Genetic Susceptibility. Journal of Clinical Medicine, 12(10), 3586. https://doi.org/10.3390/jcm12103586