Multimodality Imaging and Biomarker Approach to Characterize the Pathophysiology of Heart Failure in Left Ventricular Non-Compaction with Preserved Ejection Fraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cardiac Magnetic Resonance
- -
- mean value of native T1 for all segments (T1 global), for basal (T1 basal), mid (T1 mid), and apical (T1 apical) segments, and the gradient between apical and basal T1 (T1 base-to-apex gradient);
- -
- mean value of ECV for all segments (ECV global), for basal (ECV basal), mid (ECV mid) and apical (ECV apical) segments, and the gradient between apical and basal ECV (ECV base-to-apex gradient).
2.3. Echocardiography
2.4. Biomarkers
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Groups
3.2. Cardiac Magnetic Resonance
3.3. Regional Strain
3.4. Biomarkers
3.5. Correlations
4. Discussion
4.1. LVNC and Endothelial Dysfunction
4.2. LVNC and Myocardial Fibrosis
4.3. LVNC and Myocardial Deformation
4.4. Clinical Implications
4.5. Limitations
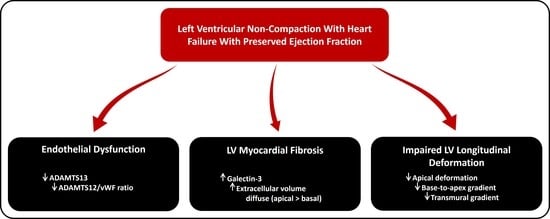
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Casas, G.; Limeres, J.; Oristrell, G.; Gutierrez-Garcia, L.; Andreini, D.; Borregan, M.; Larrañaga-Moreira, J.M.; Lopez-Sainz, A.; Codina-Solà, M.; Teixido-Tura, G.; et al. Clinical Risk Prediction in Patients with Left Ventricular Myocardial Noncompaction. J. Am. Coll. Cardiol. 2021, 78, 643–662. [Google Scholar] [CrossRef] [PubMed]
- Huttin, O.; Venner, C.; Frikha, Z.; Voilliot, D.; Marie, P.Y.; Aliot, E.; Sadoul, N.; Juillière, Y.; Brembilla-Perrot, B.; Selton-Suty, C. Myocardial deformation pattern in left ventricular non-compaction: Comparison with dilated cardiomyopathy. IJC Heart Vasc. 2014, 5, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Akhan, O.; Demir, E.; Dogdus, M.; Cakan, F.O.; Nalbantgil, S. Speckle tracking echocardiography and left ventricular twist mechanics: Predictive capabilities for noncompaction cardiomyopathy in the first degree relatives. Int. J. Cardiovasc. Imaging 2021, 37, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Anwer, S.; Heiniger, P.S.; Rogler, S.; Erhart, L.; Cassani, D.; Kuzo, N.; Rebellius, L.; Schoenenberger-Berzins, R.; Schmid, D.; Nussbaum, S.; et al. Left ventricular mechanics and cardiovascular outcomes in non-compaction phenotype. Int. J. Cardiol. 2021, 336, 73–80. [Google Scholar] [CrossRef]
- Szűcs, A.; Kiss, A.R.; Gregor, Z.; Horváth, M.; Tóth, A.; Dohy, Z.; Szabó, L.E.; Suhai, F.I.; Merkely, B.; Vágó, H. Changes in strain parameters at different deterioration levels of left ventricular function: A cardiac magnetic resonance feature-tracking study of patients with left ventricular noncompaction. Int. J. Cardiol. 2021, 331, 124–130. [Google Scholar] [CrossRef]
- Sedmera, D.; Pexieder, T.; Vuillemin, M.; Thompson, R.P.; Anderson, R.H. Developmental patterning of the myocardium. Anat. Rec. 2000, 258, 319–337. [Google Scholar] [CrossRef]
- Wu, M. Mechanisms of Trabecular Formation and Specification During Cardiogenesis. Pediatr. Cardiol. 2018, 39, 1082–1089. [Google Scholar] [CrossRef]
- Tian, X.; Li, Y.; He, L.; Zhang, H.; Huang, X.; Liu, Q.; Pu, W.; Zhang, L.; Li, Y.; Zhao, H.; et al. Identification of a hybrid myocardial zone in the mammalian heart after birth. Nat. Commun. 2017, 8, 87. [Google Scholar] [CrossRef]
- Wengrofsky, P.; Armenia, C.; Oleszak, F.; Kupferstein, E.; Rednam, C.; Mitre, C.A.; McFarlane, S.I. Left Ventricular Trabeculation and Noncompaction Cardiomyopathy: A Review. EC Clin. Exp. Anat. 2019, 2, 267–283. [Google Scholar]
- Jenni, R.; Oechslin, E.; Schneider, J.; Attenhofer Jost, C.; Kaufmann, P.A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart 2001, 86, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Junga, G.; Kneifel, S.; Smekal, A.V.; Steinert, H.; Bauersfeld, U. Myocardial ischaemia in children with isolated ventricular non-compaction. Eur. Heart J. 1999, 20, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Rodríguez, E.; Monserrat, L.; Alvarez, N. MRI of subendocardial perfusion deficits in isolated left ventricular noncompaction. J. Comput. Assist. Tomogr. 2002, 26, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Jenni, R.; Wyss, C.A.; Oechslin, E.N.; Kaufmann, P.A. Isolated ventricular noncompaction is associated with coronary microcirculatory dysfunction. J. Am. Coll. Cardiol. 2002, 39, 450–454. [Google Scholar] [CrossRef]
- De Melo, M.D.T.; Giorgi, M.C.P.; Assuncao, A.N.; Dantas, R.N.; De Arimateia Araujo Filho, J.; Filho, J.R.P.; De Souza Bierrenbach, A.L.; De Lima, C.R.; Soares, J.; Meneguetti, J.C.; et al. Decreased glycolytic metabolism in non-compaction cardiomyopathy by 18F-fluoro-2-deoxyglucose positron emission tomography: New insights into pathophysiological mechanisms and clinical implications. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 915–921. [Google Scholar] [CrossRef]
- Vancheri, F.; Longo, G.; Vancheri, S.; Henein, M. Coronary Microvascular Dysfunction. J. Clin. Med. 2020, 9, 2880. [Google Scholar] [CrossRef]
- Kalavakunta, J.K.; Tokala, H.; Gosavi, A.; Gupta, V. Left ventricular noncompaction and myocardial fibrosis: A case report. Int. Arch. Med. 2010, 3, 2–5. [Google Scholar] [CrossRef]
- Araujo-Filho, J.A.B.; Assuncao, A.N.; Tavares De Melo, M.D.; Bière, L.; Lima, C.R.; Dantas, R.N.; Nomura, C.H.; Salemi, V.M.C.; Jerosch-Herold, M.; Parga, J.R. Myocardial T1 mapping and extracellular volume quantification in patients with left ventricular non-compaction cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 888–895. [Google Scholar] [CrossRef]
- Stöllberger, C.; Gerecke, B.; Finsterer, J.; Engberding, R. Refinement of echocardiographic criteria for left ventricular noncompaction. Int. J. Cardiol. 2013, 165, 463–467. [Google Scholar] [CrossRef]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Neubauer, S. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef]
- Jacquier, A.; Thuny, F.; Jop, B.; Giorgi, R.; Cohen, F.; Gaubert, J.Y.; Vidal, V.; Bartoli, J.M.; Habib, G.; Moulin, G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur. Heart J. 2010, 31, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; Von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J. Cardiovasc. Magn. Reson. 2013, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Radjenovic, A.; Kozerke, S.; Higgins, D.M.; Sivananthan, M.U.; Ridgway, J.P. Modified look-locker inversion recovery (MOLLI) for high-resolution T 1 mapping of the heart. Magn. Reson. Med. 2004, 52, 141–146. [Google Scholar] [CrossRef]
- Schelbert, E.B.; Testa, S.M.; Meier, C.G.; Ceyrolles, W.J.; Levenson, J.E.; Blair, A.J.; Kellman, P.; Jones, B.L.; Ludwig, D.R.; Schwartzman, D.; et al. Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: Slow infusion versus bolus. J. Cardiovasc. Magn. Reson. 2011, 13, 16. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Victor, M.A.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Goncharov, N.V.; Nadeev, A.D.; Jenkins, R.O.; Avdonin, P.V. Markers and Biomarkers of Endothelium: When Something Is Rotten in the State. Oxid. Med. Cell. Longev. 2017, 2017, 9759735. [Google Scholar] [CrossRef]
- Reardon, B.; Pasalic, L.; Favaloro, E.J. The Intriguing Relationships of von Willebrand factor, ADAMTS13 and Cardiac Disease. J. Cardiovasc. Dev. Dis. 2021, 8, 115. [Google Scholar] [CrossRef]
- Sonneveld, M.A.H.; De Maat, M.P.M.; Leebeek, F.W.G. Von Willebrand factor and ADAMTS13 in arterial thrombosis: A systematic review and meta-analysis. Blood Rev. 2014, 28, 167–178. [Google Scholar] [CrossRef]
- Sygitowicz, G.; Maciejak-Jastrzębska, A.; Sitkiewicz, D. The diagnostic and therapeutic potential of galectin-3 in cardiovascular diseases. Biomolecules 2022, 12, 46. [Google Scholar] [CrossRef]
- Perea, R.J.; Morales-Ruiz, M.; Ortiz-Perez, J.T.; Bosh, X.; Andreu, D.; Borras, R.; Acosta, J.; Penela, D.; Prat-González, S.; de Caralt, T.M.; et al. Utility of galectin-3 in predicting post-infarct remodeling after acute myocardial infarction based on extracellular volume fraction mapping. Int. J. Cardiol. 2016, 223, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, G.; Del Franco, A.; Giannoni, A.; Prontera, C.; Ripoli, A.; Barison, A.; Masci, P.G.; Aquaro, G.D.; Solal, A.C.; Padeletti, L.; et al. Galectin-3 and myocardial fibrosis in nonischemic dilated cardiomyopathy. Int. J. Cardiol. 2015, 184, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lin, X.; Fang, L.; Zhao, X.; Ding, H.; Chen, W.; Xu, R.; Bai, X.; Wang, Y.; Fang, Q. Characterization of compacted myocardial abnormalities by cardiac magnetic resonance with native T1 mapping in left ventricular non-compaction patients: A comparison with late gadolinium enhancement. Circ. J. 2016, 80, 1210–1216. [Google Scholar] [CrossRef]
- Szemraj, J.; Masiarek, K.; Majos, A.; Szemraj-Rogucka, Z.M. Circulating microRNAs as biomarkers for myocardial fibrosis in patients with left ventricular non-compaction cardiomyopathy. Arch. Med. Sci. 2019, 15, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Grothoff, M.; Pachowsky, M.; Hoffmann, J.; Posch, M.; Klaassen, S.; Lehmkuhl, L.; Gutberlet, M. Value of cardiovascular MR in diagnosing left ventricular non-compaction cardiomyopathy and in discriminating between other cardiomyopathies. Eur. Radiol. 2012, 22, 2699–2709. [Google Scholar] [CrossRef]
- Asmakutlu, O.; Alis, D.; Topel, C.; Sahin, A. Late gadolinium enhancement on CMRI in patients with LV noncompaction: An overestimated phenomenon? Clin. Imaging 2020, 66, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, G.; Aquaro, G.D.; Pingitore, A.; Masci, P.G.; Lombardi, M. Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur. J. Heart Fail. 2011, 13, 170–176. [Google Scholar] [CrossRef]
- Rogucka, Z.S.; Majos, A. Left Ventricular Non-Compaction: Mid-myocardial Distribution of Late Gadolinium Enhancement in Compacted Segments. OMI J. Radiol. 2017, 6, 6–11. [Google Scholar] [CrossRef]
- Eijgenraam, T.R.; Silljé, H.H.W.; de Boer, R.A. Current understanding of fibrosis in genetic cardiomyopathies. Trends Cardiovasc. Med. 2020, 30, 353–361. [Google Scholar] [CrossRef]
- Ottaviani, G.; Segura, A.M.; Rajapreyar, I.N.; Zhao, B.; Radovancevic, R.; Loyalka, P.; Kar, B.; Gregoric, I.; Buja, L.M. Left ventricular noncompaction cardiomyopathy in end-stage heart failure patients undergoing orthotopic heart transplantation. Cardiovasc. Pathol. 2016, 25, 293–299. [Google Scholar] [CrossRef]
- Guigui, S.A.; Horvath, S.A.; Arenas, I.A.; Mihos, C.G. Cardiac geometry, function and mechanics in left ventricular non-compaction cardiomyopathy with preserved ejection fraction. J. Echocardiogr. 2022, 20, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, D.; Michelena, H.I.; Martinez, M.; Pellikka, P.A.; Bruce, C.J.; Connolly, H.M.; Villarraga, H.R.; Veress, G.; Oh, J.K.; Miller, F.A.; et al. Speckle myocardial imaging modalities for early detection of myocardial impairment in isolated left ventricular non-compaction. Heart 2010, 96, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Haland, T.F.; Saberniak, J.; Leren, I.S.; Edvardsen, T.; Haugaa, K.H. Echocardiographic comparison between left ventricular non-compaction and hypertrophic cardiomyopathy. Int. J. Cardiol. 2017, 228, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Tarando, F.; Coisne, D.; Galli, E.; Rousseau, C.; Viera, F.; Bosseau, C.; Habib, G.; Lederlin, M.; Schnell, F.; Donal, E. Left ventricular non-compaction and idiopathic dilated cardiomyopathy: The significant diagnostic value of longitudinal strain. Int. J. Cardiovasc. Imaging 2017, 33, 83–95. [Google Scholar] [CrossRef] [PubMed]




| LVNC (n = 21) | Control (n = 21) | p-Value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 61.5 ± 8.7 | 66.0 ± 6.3 | 0.06 |
| Female gender (%) | 81.0 | 85.7 | 0.67 |
| BMI (kg/m2) | 30.2 ± 5.7 | 29.3 ± 3.9 | 0.55 |
| Heart rate (bpm) | 66.6 ± 10.3 | 66.0 ± 10.8 | 0.85 |
| Systolic blood pressure (mmHg) | 142.7 ± 17.3 | 138.1 ± 22.5 | 0.46 |
| Smoking (%) | 23.8 | 14.3 | 0.43 |
| Obesity (%) | 47.6 | 42.9 | 0.76 |
| Hypertension (%) | 90.5 | 95.2 | 0.54 |
| Dyslipidaemia (%) | 90.5 | 85.7 | 0.63 |
| Diabetes mellitus (%) | 28.6 | 33.3 | 0.73 |
| CKD # (%) | 4.8 | 9.5 | 0.55 |
| History of AFib (%) | 9.5 | 14.3 | 0.64 |
| LV volumes and systolic function parameters | |||
| LVEDViCMR (mL/m2) | 77.8 ± 12.8 | 70.2 ± 10.9 | 0.04 |
| LVESViCMR (mL/m2) | 30.9 ± 9.0 | 26.7 ± 5.3 | 0.07 |
| LVEFCMR (%) | 60.8 ± 6.5 | 62.3 ± 5.3 | 0.40 |
| LVEFTTE (%) | 59.7 ± 5.2 | 60.3 ± 4.7 | 0.69 |
| LS | −19.3 ± 2.7 | −20.2 ± 2.2 | 0.25 |
| Structural and diastolic function parameters | |||
| LVMI (g/m2) | 101.4 ± 21.8 | 103.1 ± 30.2 | 0.84 |
| Relative wall thickness | 0.46 ± 0.07 | 0.50 ± 0.06 | 0.10 |
| LAVI (mL/m2) * | 39.7 (15.2) | 39.5 (8.7) | 0.97 |
| PASP (mmHg) * | 34.0 (5.5) | 33.0 (9.0) | 0.40 |
| E/e’ ratio | 10.2 ± 3.8 | 11.4 ± 2.7 | 0.26 |
| Biomarkers | |||
| NT-proBNP (pg/mL) * | 237 (156–489) | 156 (139–257) | 0.04 |
| Galectin-3 (ng/mL) * | 7.3 (6.0–11.5) | 5.6 (4.8–8.3) | 0.04 |
| ADAMTS13 (ng/mL) | 767.3 ± 335.5 | 962.3 ± 253.7 | 0.04 |
| vWF (ng/mL) * | 25.2 (23.1–30.1) | 24.0 (21.4–26.3) | 0.16 |
| ADAMTS13/vWF ratio * | 31.3 (14.8–42.3) | 40.8 (32.0–52.5) | 0.03 |
| Parameter | LVNC (n = 21) | Control (n = 21) | p-Value |
|---|---|---|---|
| Native T1 | |||
| T1 global (ms) | 1014 ± 32 | 1003 ± 28 | 0.26 |
| T1 basal (ms) | 1003 ± 27 | 1004 ± 29 | 0.82 |
| T1 mid (ms) | 997 ± 36 | 999 ± 31 | 0.82 |
| T1 apical (ms) | 1061 ± 72 | 1008 ± 40 | 0.005 |
| T1 base-to-apex gradient (ms) * | 41 (23–86) | 2.5 (−28–28) | 0.002 |
| ECV | |||
| ECV global (%) | 27.2 ± 2.9 | 24.4 ± 2.5 | 0.002 |
| ECV basal (%) | 26.2 ± 2.9 | 24.3 ± 2.6 | 0.03 |
| ECV mid (%) | 26.6 ± 3.3 | 23.8 ± 2.6 | 0.005 |
| ECV apical (%) | 29.6 ± 3.8 | 25.2 ± 2.8 | <0.001 |
| ECV base-to-apex gradient (%) * | 2.8 (1.2–5.6) | 0.9 (0.1–2.1) | 0.01 |
| ECV by segments | |||
| Segment 1: basal anterior (%) | 25.1 ± 3.3 | 23.0 ± 3.0 | 0.03 |
| Segment 2: basal anteroseptal (%) | 26.4 ± 3.5 | 25.7 ± 3.7 | 0.50 |
| Segment 3: basal inferoseptal (%) | 26.7 ± 4.1 | 23.6 ± 2.4 | 0.005 |
| Segment 4: basal inferior (%) | 26.4 ± 3.7 | 23.8 ± 3.1 | 0.02 |
| Segment 5: basal inferolateral (%) | 26.3 ± 3.4 | 25.2 ± 3.8 | 0.31 |
| Segment 6: basal anterolateral (%) | 26.1 ± 4.3 | 24.3 ± 3.6 | 0.17 |
| Segment 7: mid-anterior (%) | 25.9 ± 3.7 | 23.4 ± 2.6 | 0.01 |
| Segment 8: mid-anteroseptal (%) | 26.5 ± 3.1 | 24.0 ± 2.8 | 0.01 |
| Segment 9: mid-inferoseptal (%) | 26.7 ± 3.7 | 23.9 ± 2.6 | 0.01 |
| Segment 10: mid-inferior (%) | 25.9 ± 4.2 | 23.0 ± 2.9 | 0.01 |
| Segment 11: mid-inferolateral (%) | 27.1 ± 5.6 | 24.5 ± 3.4 | 0.07 |
| Segment 12: mid-anterolateral (%) | 27.6 ± 4.3 | 24.1 ± 4.0 | 0.01 |
| Segment 13: apical anterior (%) | 30.5 ± 4.4 | 25.2 ± 2.9 | <0.001 |
| Segment 14: apical septal (%) | 29.0 ± 3.7 | 24.9 ± 2.8 | <0.001 |
| Segment 15: apical inferior (%) | 28.7 ± 4.8 | 24.7 ± 3.4 | 0.004 |
| Segment 16: apical lateral (%) | 30.2 ± 5.2 | 25.9 ± 3.3 | 0.004 |
| Parameter | LVNC (n = 21) | Control (n = 21) | p-Value |
|---|---|---|---|
| Strain by levels | |||
| LS basal (%) | −17.5 ± 2.6 | −17.3 ± 2.4 | 0.82 |
| LS mid (%) | −18.9 ± 2.6 | −18.7 ± 2.5 | 0.79 |
| LS apical (%) | −21.4 ± 4.4 | −24.3 ± 3.2 | 0.01 |
| LS base-to-apex gradient (%) | 3.8 ± 4.7 | 6.9 ± 3.4 | 0.02 |
| Strain by layers | |||
| LS endo (%) | −21.4 ± 2.7 | −22.7 ± 2.6 | 0.11 |
| LS epi (%) | −17.4 ± 2.5 | −17.9 ± 1.9 | 0.50 |
| LS transmural gradient (%) | 3.9 ± 0.8 | 4.8 ± 1.0 | 0.006 |
| Strain by segments | |||
| Segment 1: basal anterior (%) | −16.0 ± 3.9 | −18.1 ± 3.6 | 0.07 |
| Segment 2: basal anteroseptal (%) | −16.4 ± 5.4 | −15.6 ± 3.1 | 0.58 |
| Segment 3: basal inferoseptal (%) | −13.9 ± 3.1 | −13.7 ± 3.4 | 0.85 |
| Segment 4: basal inferior (%) | −20.1 ± 4.4 | −17.4 ± 3.2 | 0.03 |
| Segment 5: basal inferolateral (%) | −20.5 ± 5.0 | −19.5 ± 4.0 | 0.48 |
| Segment 6: basal anterolateral (%) | −18.2 ± 4.0 | −19.2 ± 3.1 | 0.19 |
| Segment 7: mid-anterior (%) | −16.1 ± 4.3 | −17.5 ± 3.4 | 0.27 |
| Segment 8: mid-anteroseptal (%) | −20.9 ± 3.7 | −20.5 ± 4.3 | 0.79 |
| Segment 9: mid-inferoseptal (%) | −17.9 ± 3.1 | −18.4 ± 3.4 | 0.61 |
| Segment 10: mid-inferior (%) | −21.8 ± 3.6 | −19.4 ± 3.2 | 0.03 |
| Segment 11: mid-inferolateral (%) | −18.8 ± 3.3 | −18.6 ± 3.9 | 0.90 |
| Segment 12: mid-anterolateral (%) | −18.1 ± 4.1 | −17.8 ± 4.3 | 0.85 |
| Segment 13: apical anterior (%) | −20.3 ± 6.0 | −23.8 ± 4.6 | 0.04 |
| Segment 14: apical septal (%) | −22.3 ± 3.7 | −26.0 ± 3.3 | 0.002 |
| Segment 15: apical inferior (%) | −23.2 ± 5.8 | −25.2 ± 3.4 | 0.19 |
| Segment 16: apical lateral (%) | −19.6 ± 4.3 | −22.5 ± 4.0 | 0.03 |
| Segment 17: apex (%) | −21.4 ± 4.5 | −24.2 ± 3.1 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visoiu, I.-S.; Rimbas, R.C.; Nicula, A.I.; Mihaila-Baldea, S.; Magda, S.L.; Mihalcea, D.J.; Hayat, M.; Luchian, M.L.; Chitroceanu, A.M.; Vinereanu, D. Multimodality Imaging and Biomarker Approach to Characterize the Pathophysiology of Heart Failure in Left Ventricular Non-Compaction with Preserved Ejection Fraction. J. Clin. Med. 2023, 12, 3632. https://doi.org/10.3390/jcm12113632
Visoiu I-S, Rimbas RC, Nicula AI, Mihaila-Baldea S, Magda SL, Mihalcea DJ, Hayat M, Luchian ML, Chitroceanu AM, Vinereanu D. Multimodality Imaging and Biomarker Approach to Characterize the Pathophysiology of Heart Failure in Left Ventricular Non-Compaction with Preserved Ejection Fraction. Journal of Clinical Medicine. 2023; 12(11):3632. https://doi.org/10.3390/jcm12113632
Chicago/Turabian StyleVisoiu, Ionela-Simona, Roxana Cristina Rimbas, Alina Ioana Nicula, Sorina Mihaila-Baldea, Stefania Lucia Magda, Diana Janina Mihalcea, Memis Hayat, Maria Luiza Luchian, Alexandra Maria Chitroceanu, and Dragos Vinereanu. 2023. "Multimodality Imaging and Biomarker Approach to Characterize the Pathophysiology of Heart Failure in Left Ventricular Non-Compaction with Preserved Ejection Fraction" Journal of Clinical Medicine 12, no. 11: 3632. https://doi.org/10.3390/jcm12113632
APA StyleVisoiu, I. -S., Rimbas, R. C., Nicula, A. I., Mihaila-Baldea, S., Magda, S. L., Mihalcea, D. J., Hayat, M., Luchian, M. L., Chitroceanu, A. M., & Vinereanu, D. (2023). Multimodality Imaging and Biomarker Approach to Characterize the Pathophysiology of Heart Failure in Left Ventricular Non-Compaction with Preserved Ejection Fraction. Journal of Clinical Medicine, 12(11), 3632. https://doi.org/10.3390/jcm12113632