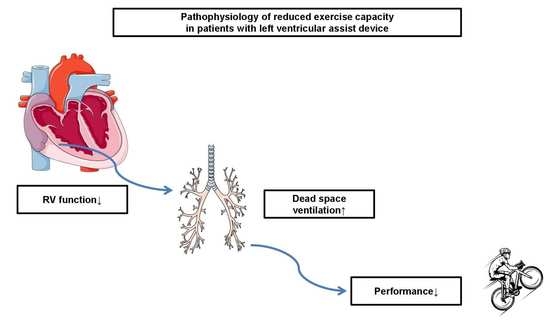
Increased Dead Space Ventilation as a Contributing Factor to Persistent Exercise Limitation in Patients with a Left Ventricular Assist Device
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. Cardiopulmonary Exercise Protocol
2.3. Co-Variable Assessment
2.4. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Bivariate Analysis of CPET Parameters between Groups
3.3. Discrimination between LVAD and HFrEF
3.4. CPET Variables to Predict the Combined Outcome
4. Discussion
4.1. Assessment of the Primary Outcome
4.2. Assessment of the Composite Secondary Outcome
4.3. Prediction of Overall Mortality
4.4. Clinical Implications of Group Differences between LVAD and HFrEF
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Baseline Variables | Min | Q25 | Q50 | Q75 | Max |
|---|---|---|---|---|---|
| Age [years] | |||||
| HFrEF (n = 108) | 24.0 | 43.0 | 53.0 | 60.0 | 80.0 |
| LVAD (n = 89) | 22.0 | 49.0 | 54.0 | 62.0 | 71.0 |
| BMI [kg/m2] | |||||
| HFrEF (n = 108) | 15.0 | 25.0 | 29.0 | 31.0 | 40.0 |
| LVAD (n = 89) | 19.0 | 25.0 | 29.0 | 31.0 | 40.0 |
| NTproBNP [pg/mL] | |||||
| HFrEF (n = 108) | 109.0 | 657.0 | 1839.0 | 4894.3 | 34,781.0 |
| LVAD (n = 89) | 35.0 | 539.5 | 937.0 | 2030.0 | 10,702.0 |
| Hb [g/dL] | |||||
| HFrEF (n = 108) | 8.5 | 12.5 | 14.6 | 15.7 | 19.7 |
| LVAD (n = 89) | 7.6 | 11.8 | 12.8 | 14.7 | 17.2 |
| Thrombocytes [/nL] | |||||
| HFrEF (n = 108) | 62.0 | 175.0 | 218.5 | 257.5 | 983.0 |
| LVAD (n = 89) | 111.0 | 183.0 | 220.0 | 277.5 | 485.0 |
| GFR [mL/min] | |||||
| HFrEF (n = 108) | 23.0 | 51.0 | 65.0 | 72.0 | 78.0 |
| LVAD (n = 89) | 26.0 | 47.5 | 60.0 | 71.0 | 76.0 |
| Echocardiographic variables | Min | Q25 | Q50 | Q75 | Max |
| LVEF [%] | |||||
| HFrEF (n = 108) | 10.0 | 20.0 | 25.0 | 30.0 | 33.0 |
| LVAD (n = 89) | 12.0 | 15.0 | 19.0 | 23.0 | 39.0 |
| TAPSE [mm] | |||||
| HFrEF (n = 108) | 6.0 | 15.0 | 18.0 | 20.8 | 28.0 |
| LVAD (n = 89) | 5.0 | 9.0 | 11.0 | 12.5 | 17.0 |
| CPET Variables | Min | Q25 | Q50 | Q75 | Max |
|---|---|---|---|---|---|
| Pmax [W] | |||||
| HFrEF (n = 108) | 25.0 | 67.3 | 94.0 | 127.8 | 202.0 |
| LVAD (n = 89) | 20.0 | 61.5 | 83.0 | 102.0 | 161.0 |
| VE [L/min] | |||||
| HFrEF (n = 108) | 25.0 | 47.0 | 61.0 | 73.0 | 107.0 |
| LVAD (n = 89) | 20.0 | 42.5 | 53.0 | 65.5 | 99.0 |
| VO2peak [mL/min/kg] | |||||
| HFrEF (n = 108) | 5.7 | 11.5 | 14.0 | 16.8 | 25.3 |
| LVAD (n = 89) | 4.3 | 11.1 | 13.1 | 15.0 | 21.8 |
| % of VO2predicted | |||||
| HFrEF (n = 108) | 17.9 | 40.2 | 52.4 | 63.9 | 86.4 |
| LVAD (n = 89) | 20.5 | 39.8 | 50.5 | 58.9 | 86.1 |
| % of VO2 at VT1 | |||||
| HFrEF (n = 108) | 10.0 | 28.0 | 34.0 | 40.0 | 56.0 |
| LVAD (n = 89) | 12.0 | 28.5 | 33.0 | 38.0 | 68.0 |
| HRmax [beats/min] | |||||
| HFrEF (n = 108) | 71.0 | 100.0 | 115.5 | 131.0 | 176.0 |
| LVAD (n = 89) | 36.0 | 101.0 | 117.0 | 129.0 | 171.0 |
| RRsysmax [mmHg] | |||||
| HFrEF (n = 108) | 74.0 | 110.0 | 130.0 | 151.8 | 260.0 |
| LVAD (n = 89) | 76.0 | 120.0 | 150.0 | 184.5 | 274.0 |
| O2 pulsemax [mL/beat/kg × 100] | |||||
| HFrEF (n = 108) | 3.9 | 8.5 | 10.9 | 13.6 | 21.6 |
| LVAD (n = 89) | 3.5 | 8.6 | 10.4 | 12.8 | 21.5 |
| OUES | |||||
| HFrEF (n = 108) | 0.3 | 1.0 | 1.4 | 1.9 | 3.1 |
| LVAD (n = 89) | 0.3 | 1.0 | 1.3 | 1.6 | 2.8 |
| VE/VCO2 | |||||
| HFrEF (n = 108) | 23.0 | 32.1 | 38.0 | 45.3 | 94.2 |
| LVAD (n = 89) | 13.2 | 30.9 | 35.6 | 41.6 | 62.5 |
| EqO2 at VT1 | |||||
| HFrEF (n = 108) | 18.0 | 23.1 | 25.7 | 30.3 | 52.0 |
| LVAD (n = 89) | 18.0 | 22.9 | 24.7 | 29.5 | 46.0 |
| Circulatory power | |||||
| [ml/kg/min × mmHg] | |||||
| HFrEF (n = 108) | 581.4 | 1329.9 | 1927.0 | 2452.2 | 4222.4 |
| LVAD (n = 89) | 567.6 | 1447.6 | 2034.9 | 2580.4 | 4438.8 |
| Ventilatory power [mmHg] | |||||
| HFrEF (n = 108) | 1.2 | 2.7 | 3.4 | 4.4 | 6.5 |
| LVAD (n = 89) | 1.8 | 3.2 | 4.6 | 5.2 | 9.5 |
| Breathing reserve [%] | |||||
| HFrEF (n = 108) | 0.0 | 26.0 | 36.5 | 50.0 | 75.0 |
| LVAD (n = 89) | 0.0 | 24.5 | 38.0 | 48.5 | 74.0 |
| VD/VT [%] | |||||
| HFrEF (n = 108) | 1.0 | 10.3 | 14.0 | 17.0 | 31.0 |
| LVAD (n = 89) | 0.0 | 14.0 | 16.0 | 18.0 | 25.0 |
| LVAD vs. HFrEF | Estimate | Standard Error | p-Value |
|---|---|---|---|
| Intercept | 6.97 | 2.47 | p < 0.01 * |
| VO2peak [mL/min/kg] | −0.04 | 0.09 | p = 0.66 |
| NTproBNP [pg/mL] | −0.01 | 0.01 | p < 0.01 * |
| TAPSE [mm] | −0.79 | 0.13 | p < 0.01 * |
| LVEF [%] | −0.01 | 0.04 | p = 0.83 |
| PETCO2 > 3 mmHg | 1.30 | 0.65 | p = 0.05 * |
| VP [mmHg] | 0.38 | 0.23 | p = 0.11 |
| VD/VT [%] | 0.23 | 0.07 | p < 0.01 * |
| Estimate | Standard Error | p-Value | |
|---|---|---|---|
| Intercept | 0.31 | 1.69 | p = 0.86 |
| Group-LVAD | 0.69 | 0.35 | p = 0.05 * |
| VO2peak [mL/min/kg] | −0.07 | 0.05 | p = 0.23 |
| VE/VCO2 | 0.04 | 0.02 | p = 0.07 |
| VP [mmHg] | −0.30 | 0.15 | p = 0.04 * |
| PETCO2 > 3 mmHg | 0.23 | 0.35 | p = 0.52 |
| VD/VT [%] | −0.04 | 0.04 | p = 0.34 |
| Estimate | Standard Error | p-Value | |
|---|---|---|---|
| Intercept | 0.31 | 1.69 | p = 0.86 |
| Group-LVAD | 0.69 | 0.35 | p = 0.05 * |
| VO2peak [mL/min/kg] | −0.07 | 0.05 | p = 0.23 |
| VE/VCO2 | 0.04 | 0.02 | p = 0.07 |
| VP [mmHg] | −0.30 | 0.15 | p = 0.04 * |
| PETCO2 > 3 mmHg | 0.23 | 0.35 | p = 0.52 |
| VD/VT [%] | −0.04 | 0.04 | p = 0.34 |
References
- Gross, C.; Marko, C.; Mikl, J.; Altenberger, J.; Schlöglhofer, T.; Schima, H.; Zimpfer, D.; Moscato, F. LVAD Pump Flow Does Not Adequately Increase With Exercise. Artif. Organs 2019, 43, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Bjarnason-Wehrens, B.; Bartsch, P.; Deniz, E.; Schmitto, J.; Schulte-Eistrup, S.; Willemsen, D.; Reiss, N. Exercise Capacity and Functional Performance in Heart Failure Patients Supported by a Left Ventricular Assist Device at Discharge From Inpatient Rehabilitation. Artif. Organs 2018, 42, 22–30. [Google Scholar] [CrossRef]
- Fresiello, L.; Jacobs, S.; Timmermans, P.; Buys, R.; Hornikx, M.; Goetschalckx, K.; Droogne, W.; Meyns, B. Limiting factors of peak and submaximal exercise capacity in LVAD patients. PLoS ONE 2020, 15, e0235684. [Google Scholar] [CrossRef]
- Mezzani, A.; Pistono, M.; Agostoni, P.; Giordano, A.; Gnemmi, M.; Imparato, A.; Temporelli, P.; Corrà, U. Exercise gas exchange in continuous-flow left ventricular assist device recipients. PLoS ONE 2018, 13, e0187112. [Google Scholar] [CrossRef] [PubMed]
- Loyaga-Rendon, R.Y.; Plaisance, E.P.; Arena, R.; Shah, K. Exercise physiology, testing, and training in patients supported by a left ventricular assist device. J. Heart Lung Transplant. 2015, 34, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Sailer, C.; Edelmann, H.; Buchanan, C.; Giro, P.; Babcock, M.; Swanson, C.; Spotts, M.; Schulte, M.; Pratt-Cordova, A.; Coe, G.; et al. Impairments in Blood Pressure Regulation and Cardiac Baroreceptor Sensitivity Among Patients With Heart Failure Supported With Continuous-Flow Left Ventricular Assist Devices. Circ. Heart Fail. 2021, 14, e007448. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Bandera, F.; Ozemek, C.; Systrom, D.; Arena, R. Cardiopulmonary Exercise Testing: What Is its Value? J. Am. Coll. Cardiol. 2017, 70, 1618–1636. [Google Scholar] [CrossRef]
- Rovai, S.; Corrà, U.; Piepoli, M.; Vignati, C.; Salvioni, E.; Bonomi, A.; Mattavelli, I.; Arcari, L.; Scardovi, A.B.; Perrone Filardi, P.; et al. Exercise oscillatory ventilation and prognosis in heart failure patients with reduced and mid-range ejection fraction. Eur. J. Heart Fail. 2019, 21, 1586–1595. [Google Scholar] [CrossRef]
- Lala, A.; Shah, K.B.; Lanfear, D.E.; Thibodeau, J.T.; Palardy, M.; Ambardekar, A.V.; McNamara, D.M.; Taddei-Peters, W.C.; Baldwin, J.T.; Jeffries, N.; et al. Predictive Value of Cardiopulmonary Exercise Testing Parameters in Ambulatory Advanced Heart Failure. JACC Heart Fail. 2021, 9, 226–236. [Google Scholar] [CrossRef]
- Forman, D.E.; Guazzi, M.; Myers, J.; Chase, P.; Bensimhon, D.; Cahalin, L.P.; Peberdy, M.A.; Ashley, E.; West, E.; Daniels, K.M.; et al. Ventilatory power: A novel index that enhances prognostic assessment of patients with heart failure. Circ. Heart Fail. 2012, 5, 621–626. [Google Scholar] [CrossRef]
- Hansen, J.E.; Sue, D.Y.; Wasserman, K. Predicted values for clinical exercise testing. Am. Rev. Respir. Dis. 1984, 129, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Kremser, C.B.; O’Toole, M.F.; Leff, A.R. Oscillatory hyperventilation in severe congestive heart failure secondary to idiopathic dilated cardiomyopathy or to ischemic cardiomyopathy. Am. J. Cardiol. 1987, 59, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, J.A.; Araújo, C.G.S.; Kurl, S.; Khan, H.; Jae, S.Y.; Guazzi, M.; Kunutsor, S.K. Relative peak exercise oxygen pulse is related to sudden cardiac death, cardiovascular and all-cause mortality in middle-aged men. Eur. J. Prev. Cardiol. 2018, 25, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. 2016 focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur. Heart J. 2018, 39, 1144–1161. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, J.A.; Savonen, K.; Hupin, D.; Araújo, C.G.S.; Kunutsor, S.K. Cardiorespiratory optimal point during exercise testing and sudden cardiac death: A prospective cohort study. Prog. Cardiovasc. Dis. 2021, 68, 12–18. [Google Scholar] [CrossRef]
- Baba, R.; Nagashima, M.; Goto, M.; Nagano, Y.; Yokota, M.; Tauchi, N.; Nishibata, K. Oxygen uptake efficiency slope: A new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J. Am. Coll. Cardiol. 1996, 28, 1567–1572. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 22 April 2022).
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmunds, L.H., Jr.; Wilson, J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Compostella, L.; Russo, N.; Setzu, T.; Compostella, C.; Bellotto, F. Exercise performance of chronic heart failure patients in the early period of support by an axial-flow left ventricular assist device as destination therapy. Artif. Organs 2014, 38, 366–373. [Google Scholar] [CrossRef]
- Wernhart, S.; Papathanasiou, M.; Jakstaite, A.; Hoffmann, J.; Schmack, B.; Hedderich, J.; Ruhparwar, A.; Rassaf, T.; Luedike, P. Exercise oscillatory ventilation in patients with advanced heart failure with and without left ventricular assist device. Artif. Organs 2022, 47, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Whipp, B.J.; Wasserman, K. Alveolar-arterial gas tension differences during graded exercise. J. Appl. Physiol. 1969, 27, 361–365. [Google Scholar] [CrossRef]
- Grinstein, J.; Sawalha, Y.; Medvedofsky, D.A.; Ahmad, S.; Hofmeyer, M.; Rodrigo, M.; Kadakkal, A.; Barnett, C.; Kalantari, S.; Talati, I.; et al. VE/VCO2 slope predicts RV dysfunction and mortality after left ventricular assist device: A fresh look at cardiopulmonary stress testing for prognostication. J. Artif. Organs 2021, 24, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Correale, M.; Monaco, I.; Ferraretti, A.; Tricarico, L.; Sicuranza, M.; Gallotta, A.M.; Formica, E.S.; Acanfora, G.; Di Biase, M.; Brunetti, N.D. Ventilatory power, a cardiopulmonary exercise testing parameter for the prediction of pulmonary hypertension at right heart catheterization. Int. J. Cardiol. Heart Vasc. 2020, 28, 100513. [Google Scholar] [CrossRef] [PubMed]
- Mirza, K.K.; Szymanski, M.K.; Schmidt, T.; de Jonge, N.; Brahmbhatt, D.H.; Billia, F.; Hsu, S.; MacGowan, G.A.; Jakovljevic, D.G.; Agostoni, P.; et al. Prognostic Value of Peak Oxygen Uptake in Patients Supported With Left Ventricular Assist Devices (PRO-VAD). JACC Heart Fail. 2021, 9, 758–767. [Google Scholar] [CrossRef]






| Medical History | HFrEF | LVAD | p-Value |
|---|---|---|---|
| (n = 108) | (n = 89) | ||
| Age [years] | 51.7 ± 10.9 | 53.6 ± 10.1 | p = 0.18 |
| BMI [kg/m2] | 28.0 ± 5.0 | 28.6 ± 4.3 | p = 0.49 |
| Women [%] | 15.7 (n = 17) | 14.6 (n = 13) | p = 0.85 |
| Diabetes [%] | 34.3 (n = 37) | 31.5% (n = 28) | p = 0.76 |
| Hypertension [%] | 44.4 (n = 48) | 46.1% (n = 41) | p = 0.89 |
| AF [%] | 23.1 (n = 25) | 48.3 (n = 43) | p < 0.01 * |
| Smoking [%] | 54.6 (n = 59) | 57.3% (n = 51) | p = 0.77 |
| CAD [%] | 50.9 (n = 55) | 66.3 (n = 59) | p = 0.03 * |
| NYHA class [%] | p = 0.55 | ||
| I | 0.9 (n = 1) | 0.0 (n = 0) | |
| II | 38.0 (n = 41) | 36.0 (n = 32) | |
| III | 57.4 (n = 62) | 62.9 (n = 56) | |
| IV | 3.7 (n = 4) | 1.1 (n = 1) | |
| Listed for heart transplant | 29.6 (n = 32) | 44.9% (n = 40) | p = 0.04 * |
| Rehospitalization [%] | 39.8% (n = 43) | 46.1% (n = 41) | p = 0.39 |
| Overall Mortality [%] | 5.6% (n = 6) | 11.2% (n = 10) | p = 0.19 |
| BB, % patients (n) | 95.4 (n = 103) | 96.6 (n = 86) | p = 0.73 |
| MRA [%] | 87.0 (n = 94) | 89.9 (n = 80) | p = 0.66 |
| ACEi/ARB/ARNI [%] | 95.4 (n = 103) | 93.3 (n = 83) | p = 0.55 |
| Loop diuretics [%] | 78.7 (n = 85) | 71.9 (n = 64) | p = 0.32 |
| SGLT2 inhibitor | 79.6 (n = 86) | 49.4 (n = 44) | p < 0.01 * |
| Laboratory values | |||
| NTproBNP [pg/mL] | 3872.2 ± 5322.4 | 1889.1 ± 2408.2 | p < 0.01 * |
| Hemoglobin [g/dl] | 14.2 ± 2.5 | 12.9 ± 2.2 | p < 0.01 * |
| eGFR [ml/min] | 59.4 ± 14.8 | 59.2 ± 14.0 | p = 0.44 |
| Thrombocytes [/nl] | 249.8 ± 274.9 | 235.2 ± 75.5 | p = 0.41 |
| Echocardiographic variables | |||
| LVEF [%] | 24.8 ± 7.8 | 20.7 ± 7.1 | p < 0.01 * |
| TAPSE [mm] | 17.4 ± 4.0 | 10.9 ± 2.3 | p < 0.01 * |
| Valve dysfunction [%] | 48.1 (n = 52) | 12.4 (n = 11) | p < 0.01 * |
| CPET Variables | HFrEF | LVAD | p-Value |
|---|---|---|---|
| (n = 108) | (n = 89) | ||
| CI [%] | 31.4 (n = 34) | 27.0 (n = 24) | p = 0.69 |
| HRmax [beats/min] | 117.5 ± 22.4 | 113.7 ± 25.2 | p = 0.57 |
| RRsysmax [mmHg] | 134.6 ± 35.3 | 155.7 ± 44.7 | p < 0.01 * |
| RER | 1.5 ± 4.3 | 1.1 ± 0.1 | p = 0.70 |
| VO2peak [mL/min/kg] | 14.3 ± 4.1 | 13.4 ± 3.5 | p = 0.11 |
| % of VO2pred | 51.9 ± 14.9 | 49.7 ± 13.0 | p = 0.33 |
| % of pred VO2 at VT1 | 34.2 ± 8.9 | 33.7 ± 8.7 | p = 0.68 |
| Pmax [W] | 97.2 ± 40.9 | 84.2 ± 31.9 | p = 0.05 * |
| VE [l] | 60.9 ± 18.5 | 54.4 ± 16.1 | p = 0.02 * |
| VE/VCO2 | 40.9 ± 12.9 | 36.7 ± 8.2 | p = 0.06 |
| VO2/W [mL/min/W] | 8.8 ± 3.3 | 8.5 ± 2.8 | p = 0.57 |
| Plateau of O2 pulse [%] | 61.1 (n = 66) | 70.8 (n = 63) | p = 0.18 |
| O2 pulsemax [mL/beat/kg × 100] | 11.1 ± 3.7 | 10.7 ± 3.3 | p = 0.36 |
| EqO2 at VT1 | 27.1 ± 5.8 | 26.2 ± 5.2 | p = 0.29 |
| OUES | 1.5 ± 0.6 | 1.4 ± 0.5 | p = 0.19 |
| VD/VT [%] | 14.2 ± 5.7 | 16.0 ± 3.9 | p < 0.01 * |
| BR FEV1 [%] | 37.3 ± 16.6 | 35.6 ± 18.9 | p = 0.74 |
| Circulatory power [mL/kg/min × mmHg] | 1933.7 ± 729.4 | 2073.2 ± 774.3 | p = 0.21 |
| Ventilatory power [mmHg] | 3.6 ± 1.3 | 4.4 ± 1.6 | p < 0.01 * |
| EOV [%] | 51.9 (n = 56) | 51.7 (n = 46) | p = 0.99 |
| PETCO2 > 3 mmHg [%] | 53.7 (n = 58) | 68.5 (n = 61) | p = 0.04 * |
| Variable | Odds Ratio | 95% Confidence Limits |
|---|---|---|
| NTproBNP [pg/mL] | 0.63 * | 0.50–0.77 |
| TAPSE [mm] | 0.45 * | 0.34–0.56 |
| PETCO2 > 3 mmHg | 4.25 * | 1.31–15.81 |
| VD/VT [%] | 1.23 * | 1.10–1.40 |
| Variable | Odds Ratio | 95% Confidence Limits |
|---|---|---|
| Group LVAD | 2.01 * | 1.07–3.85 |
| VE/VCO2 | 1.04 * | 1.00–1.08 |
| VP [mmHg] | 0.74 * | 0.55–0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wernhart, S.; Balcer, B.; Rassaf, T.; Luedike, P. Increased Dead Space Ventilation as a Contributing Factor to Persistent Exercise Limitation in Patients with a Left Ventricular Assist Device. J. Clin. Med. 2023, 12, 3658. https://doi.org/10.3390/jcm12113658
Wernhart S, Balcer B, Rassaf T, Luedike P. Increased Dead Space Ventilation as a Contributing Factor to Persistent Exercise Limitation in Patients with a Left Ventricular Assist Device. Journal of Clinical Medicine. 2023; 12(11):3658. https://doi.org/10.3390/jcm12113658
Chicago/Turabian StyleWernhart, Simon, Bastian Balcer, Tienush Rassaf, and Peter Luedike. 2023. "Increased Dead Space Ventilation as a Contributing Factor to Persistent Exercise Limitation in Patients with a Left Ventricular Assist Device" Journal of Clinical Medicine 12, no. 11: 3658. https://doi.org/10.3390/jcm12113658
APA StyleWernhart, S., Balcer, B., Rassaf, T., & Luedike, P. (2023). Increased Dead Space Ventilation as a Contributing Factor to Persistent Exercise Limitation in Patients with a Left Ventricular Assist Device. Journal of Clinical Medicine, 12(11), 3658. https://doi.org/10.3390/jcm12113658