Cellular and Molecular Evidence of Multiple Sclerosis Diagnosis and Treatment Challenges
Abstract
:1. Introduction
2. MS Epidemiological Status
3. AetiologicalFactors Responsible for MS
3.1. Epstein-Barr Virus (EBV)
3.2. Vitamin Ddeficiency
3.3. Genes
3.4. Tobacco Use
3.5. Adolescent Obesity
3.6. Females Are More Susceptible to MS
3.7. Immune Responses
4. Diagnostic Challenges
4.1. Misdiagnosis
4.2. MS Misinterpretation
4.3. Biomarker Development Challenges
4.4. Treatment Costs
5. Currently Available Diagnoses of MS
5.1. CSF andSerum
5.1.1. Oligoclonal Bands (IgG) and (IgM)
5.1.2. NO (Nitric Oxide)
5.1.3. Serum Glial Fibrillary Acidic Protein (sGFAP)
5.2. Saliva and Serum
MBP (Myelin Basic Protein)
5.3. Urine Metabolites
5.4. Tear
5.4.1. Alpha-1 Antichymotrypsin
5.4.2. OCB
5.5. Predictive Biomarkers
5.5.1. HHV-6
5.5.2. Antibodies against MOG and MBP
5.6. PrognosticBiomarkers
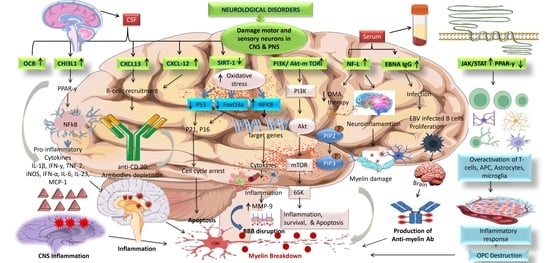
5.6.1. Chitinase-3-Like-1 (CHI3L1)
5.6.2. Neurofilaments
5.6.3. MicroRNAs (miRNAs)
5.6.4. CXCL13
5.6.5. CXCL12
5.6.6. SIRT-1
5.6.7. PI3K/AKT/mTOR
5.6.8. EBNA igG
5.6.9. JAK/STAT and PPAR-γ
| S.N. | Biomarkers | Biological Samples | Ranges (Units) | Types/ Category of MS Patients | Patients | References | |
|---|---|---|---|---|---|---|---|
| MS | Normal ranges | Age and Gender | |||||
| 1. | Chitinase-3-like-1(CHI3L1) | CSF | 22.7 pg/mL | 13.4–57.9 pg/mL | RRMS | Mean Age: 30.3 ± 9.25 years Gender: 46Fand13M | [191] |
| Serum | 20.2 pg/mL | 9.8–75.9 pg/mL | CIS | ||||
| 2. | CXCL13 | CSF | 35 mg/dL | <30 mg/dL | RRMS | MeanAge:34 ± 8.3 years Gender: 5F and3M | [191] |
| 3. | Neurofilament | Plasma | 11.4 pg/mL | 7.5 pg/mL | RRMS | Mean age: 40 Gender: 328F and 344M | [192] |
| 4. | EBNA1 IgG | Serum | 310.91 U/mL | 177.81 U/mL | RRMS | Mean age: 29.69 Gender: 27M and 48F | [193] |
| 5. | OCBs | CSF | 5–7 bands | 1 band | RRMS, SPMS | Mean Age: 35 Gender: 14F and 8M | [194,195] |
| 6. | miRNA | Plasma | ±>1.5 fold change | −5.30 to +1.94 Fold range | RRMS | Mean Age: 31.5 Gender: 20F and 16M | [196] |
| 7. | Alpha-1 antichymotrypsin | Tears | 1.6 ng/L | 2.5 ng/L | RRMS = 25, PPMS = 4, SPMS = 1 | Mean age: 42.4 ± 15 Gender: Males | [141] |
| 8. | Myelinbasic protein (MBP) | Serum | 1055 ng/L | 2750 ng/L | RRMS | Mean age: 36.8 ± 4.2 Gender: Females | [129] |
| Saliva | 475 ng/L | 575 ng/L | RRMS | ||||
6. Treatment Challenges
6.1. Inadequate Treatment Initiation
6.2. Lack of Continuous Treatment
6.3. Patients with Concomitant Liver Illness or a History of Drug-Induced Liver Damage
6.4. Elderly Patients
6.5. Pregnancy and Family Planning
| S.NO | Nameof Drugs | Dose and Route | Adverse Effect | Patients Type | Duration of Treatment | References |
|---|---|---|---|---|---|---|
| 1. | Fingolimod (Peptide) | 0.5 mg p.o. daily | Infections, bradycardia, MS relapse, and basal-cell carcinoma. | RRMS | 6–12 months | [239,240] |
| 2. | Interferon β-1a (Glycoprotein) | 30 mcg (IM), Once a day 22 mcg (SC), TDI | Flu-like symptoms (fever, chills, sweating, muscleaches, and tiredness), skin reaction, depression, anxiety, and liver problems. | RRMS | 24 months | [100,241,242] |
| 3. | Interferon β-1a (Glycoprotein) | 22 mg, three injections weekly (SC) | Fatigue, allergic reactions, flu-like symptoms, emotional instability, trouble breathing, joint problem, eye problems, and hair loss. | RRMS | 6–24 months | [241,243,244] |
| 4. | Interferon β-1b (Non-glycosylated protein) | 0.25 mg (SC) q.o.d., 6 weeks | Leucopenia, flu-like symptoms, elevated hepatic transaminases, injection site reactions, headache, fever, malaise, and myalgia. | RRMS | 24 months | [242,245,246] |
| 5. | Alemtuzumab (Monoclonal antibody) | 12 mg (IV) daily | Infusion-associated reactions(IARs) include headache, rash, nausea, fever, respiratory tract infection, and thyroid disease. | RRMS | 12 months | [247,248] |
| 6. | Dimethyl Fumarate (Peptide) | 240 mg/kg (p.o.) Twice a day | Abdominal pain, alopecia, back pain, cough, diarrhea, flushing, headache, influenza, paresthesia, and nausea. | RRMS | 24 weeks | [249] |
| 7. | Glatiramer acetate (peptide) | 20 mg/kg (SC) daily | Post-injection reaction, chest pain, lipoatrophy, and skin necrosis potentially affect the immune response. | RRMS | 24 months | [250,251]. |
| 8. | Dalfampridine (Pyrimidine analogue) | 10 mg/kg twice a day | Asthenia, insomnia, paresthesia, UTI, dizziness, nausea, peripheral edema, back pain, and nasopharyngitis. | RRMS | 4–24 weeks | [252,253] |
| 9. | Natalizumab (Monoclonal antibody) | 300 mg/kg (i.v.) | Occurrence of PML, fatal cases of neutralizing antibodies, and PML HSV1/VZV reactivation. | RRMS | ≥12 months | [202,250]. |
| 10. | Ocrelizumab (Monoclonal antibody) | 300 mg/kg (i.v.) | HSV1/VZV reactivation, HBV hypogammaglobulinemia, and breastcancer PML (carry over). | RRMS | 6 months | [250,254] |
| 11. | Teriflunomide (Enamide) | 14 mg/kg (p.o.) | Hepatic events, lymphopenia, neutropenia, thrombocytopenia, hypertension, pancreatic disorders, hair thinning, and GIT events. | RRMS | 12 weeks | [255,256] |
| 12. | Siponimod (Alkoxyimino) | 0.25–2 mg/kg (p.o.) | Bradycardia, rapid receptor desensitization, decreased absolute lymphocyte count (ALC), lymphopenia, upper respiratory tract infections, pharyngitis, insomnia, and increased alanine aminotransferase. | RRMS | >12 months | [257,258] |
| 13. | Rituximab (Chimeric murine/human monoclonal antibody) | 500–1000 mg (IV) | Infusion-related adverse events include rash, fatigue, chills, nausea, and general pain. | RRMS | 72 weeks | [259,260] |
| 14. | Mitoxantrone (dihydroxyanthraquinone) | 12 mg/kgbody weight every three months | Mild infections, leucopenia, irreversible amenorrhea, congestive heart failure, alopecia, and asymptomatic systolic dysfunction. | RRMS and SPMS | 2–3 years | [261] |
| 15. | Azathioprine (Purine analogue) | 3 mg/kg daily (p.o.) | GIT disturbance, hepatic toxicity, bone marrow suppression, hepatic toxicity, and increased risk of cancer in MS patients. | RRMS | 6 months | [262] |
| 16. | Methylprednisolone (Corticosteroids) | 500–1000 mg/daily Oral/i.v. | It may cause interaction with warfarin, reduce the effects of enzyme inducers like anti-epileptic agents, dyspepsia, constipation, euphoria, and altered glucose metabolism. | RRMS | 3–5 days | [263] |
| 17. | Cladribine (Purine antimetabolite) | 3.5 mg/kg (p.o.) two times, 4 or 5 days of treatment each year | Mild renal impairment, hepatic impairment, contraindicated in patients with moderate or severe renal impairment (creatinine clearance < 60 mL/min), and lymphopenia. | RRMS | 2 years | [264,265] |
| 18. | Simvastatin (Statin) | 80 mg/kg, per day (p.o.) | Muscle pain, dizziness, fainting, headache, nausea, and digestive problems. | SPMS | 24 months | [266,267] |
| 19. | Memantine (Amine) | 20 mg/day | Headache, dizziness, agitation, hallucinations, confusion, and diarrhea. | RRMS | 52 weeks | [268,269] |
| 20. | Donepezil (Peptide) | 10 mg/daily (p.o.) | Nausea, diarrhea, headaches, gastroesophageal reflexes, and loss of appetite. | RRMS | 24 weeks | [270,271] |
| 21. | Baclofen (Peptide) | 10–100 mcg intrathecal | Dizziness, drowsiness, headache, weakness, and nausea. | SPMS and PPMS | 4.9 years | [272] |
| 22. | Ublituximab (Monoclonal antibody) | 150–600 mg/kg i.v. infusion | Infusion-related reactions, nausea, upper respiratory tract infection, arthralgia, hypoesthesia, dizziness, fatigue, and diarrhea. | RRMS and SPMS | 48 weeks | [273] |
| 23. | Ponesimod (Peptide) | 10,20, 40 mg/kg Daily (p.o.) | Increase alanine aminotransferase, nasopharyngitis, headache, upper respiratory tract infections, and alopecia. | RRMS and SPMS | 24 weeks | [274,275] |
| 24. | Ofatumumab (Monoclonal antibody) | 20 mg/kg (S.C.) | Headache, nasopharyngitis, urinary tract infections (UTI), upper respiratory infections, and injection-site reactions (pain, itching, erythema, and swelling). | RRMS and SPMS | 12 weeks | [276] |
| 25. | Monomethyl Fumarate (Non-peptide) | 95–190 mg/kg (b.i.d.), orally Delayed release capsule | Flushing and GI adverse events (abdominal pain, diarrhea, nausea, and vomiting). | RRMS and SPMS | 5 weeks | [277] |
| 26. | Laquinimod (Amide) | 0.3–0.6 mg/kg (p.o.) | Elevation of liver enzymes, back pain, abdominal pain, cough, dizziness, headache, diarrhea, and respiratory pain. | RRMS and PPMS | 12–24 months | [278,279] |
7. Future Perspectives
7.1. Early Diagnosis of MS
- Follow-up with the MS patient
- Referrals to neuroscientists from other doctors
- Modern Diagnostic Techniques Adoption
- Exclusion of illnesses with comparable clinical manifestations
- Early detection and changing diagnostic criteria
- Increased Education and Awareness
- Collaborative Investigation
7.2. For the Treatment of MS
- Priority will be given to counseling
- Employment of improved imaging techniques
- Developing new disease-modifying therapies
- Infrastructure expansion in healthcare
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AQP4 | Aquaporin-4 |
| CXCL13 | C-X-C Motif chemokine ligament |
| CHI3L1 | Chitinase-3-like-1 |
| CDMS | Clinically definitive MS |
| CIS | Clinically isolated syndrome |
| COI | Cost of illness |
| DMT | Disease-modifying therapy |
| DMA | Disease-modifying agents |
| DMDs | Disease-modifying drugs |
| DILI | Drug-induced liver damage |
| EDSS | Expanded disability status scale |
| EMA | European medicine agency |
| EBA | Epstein-Barr virus |
| GDP | Gross domestic product |
| GFAB | Glial Fibrillary acidic protein |
| GA | Glatiramer acetate |
| HLA | Human leukocyte antigen |
| HHV-6 | Human herpes virus-6 |
| ILs | Interleukins |
| IFN-γ | Interferon-gamma |
| IRDA | Insurance regulatory anddevelopment authority of India |
| iNOS | Inducible-type nitric oxide |
| IF | Intermediate filament |
| IBD | Inflammatory bowel disease |
| MS | Multiple sclerosis |
| MRI | Magnetic resonance imaging |
| MHC | Major histocompatibility complex |
| MOG | Myelin oligodendrocytes |
| MAGNIMS | Magnetic resonance imaging in MS |
| NEDA | No evidence of disease activity |
| NMOSD | Neuromyelitisoptica spectrum disease |
| NMO | Neuromyelitis optica |
| NR | Neurology resident |
| NO | Nitric oxide |
| NFL | Neurofilament light chain |
| ONTT | Optic neuritis |
| OCB | Oligoclonal bands |
| OCGB | Patched |
| ON | Optic neuritis |
| OCT | Optical coherence tomography |
| PPMS | Primary progressive MS |
| PET | positron emission tomography |
| RRMS | Relapsing-remitting MS |
| RIS | Radiologically isolated syndrome |
| SPMS | Secondary progressive MS |
| SNPs | Single-nucleotide polymorphism |
| TNF-α | Tumor necrosis factor-alpha |
| Th | T-helper cell |
| WHO | World Health Organization |
References
- Kumar, N.; Sahoo, N.K.; Mehan, S.; Verma, B. The importance of gut-brain axis and use of probiotics as a treatment strategy for multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 71, 104547. [Google Scholar] [CrossRef] [PubMed]
- Pachner, A. The Brave New World of Early Treatment of Multiple Sclerosis: Using the Molecular Biomarkers CXCL13 and Neurofilament Light to Optimize Immunotherapy. Biomedicines 2022, 10, 2099. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rohatgi, A.; Chaudhari, H.; Thakor, P. Evolving landscape of multiple sclerosis in India: Challenges in the management. Ann. Indian Acad. Neurol. 2018, 21, 107. [Google Scholar] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Bowne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef] [Green Version]
- Singhal, B.; Ganeshan, M. Multiple Sclerosis: The disease and its burden. In Clinical Practice of Multiple Sclerosis; Srivastava, M.V.P., Bhatia, R., Eds.; Kontentworx Communications: New Delhi, India, 2014; pp. 1–12. [Google Scholar]
- Kearns, P.K.A.; Paton, M.; O’Neill, M.; Waters, C.; Colville, S.; McDonald, J.; Young, I.J.B.; Pugh, D.; O’Riordan, J.; Weller, B.; et al. Regional variation in the incidence rate and sex ratio of multiple sclerosis in Scotland 2010-2017: Findings from the Scottish Multiple Sclerosis Register. J. Neurol. 2019, 266, 2376–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenager, E. A global perspective on the burden of multiple sclerosis. Lancet Neurol. 2019, 18, 227–228. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, T.; Mehan, S. Neuroprotective Methodologies in the Treatment of Multiple Sclerosis Current Status of Clinical and Pre-clinical Findings. Curr. Drug Discov. Technol. 2021, 18, 31–46. [Google Scholar] [CrossRef]
- Zahoor, I.; Haq, E. Multiple sclerosis in India: Iceberg or volcano. J. Neuroimmunol. 2017, 307, 27–30. [Google Scholar] [CrossRef]
- Singhal, B.S. Multiple sclerosis--Indian experience. Ann. Acad. Med. 1985, 14, 32–36. [Google Scholar]
- Zahoor, I.; Asimi, R.; Haq, E.; Wani, I.Y. Demographic and clinical profile of Multiple Sclerosis in Kashmir: A short report. Mult. Scler. Relat. Disord. 2017, 13, 103–106. [Google Scholar] [CrossRef]
- Eskandarieh, S.; Heydarpour, P.; Minagar, A.; Pourmand, S.; Sahraian, M.A. Multiple sclerosis epidemiology in east Asia, south east Asia and south Asia: A systematic review. Neuroepidemiology 2016, 46, 209–221. [Google Scholar] [CrossRef]
- GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer-Gould, A.; Brara, S.M.; Beaber, B.E.; Zhang, J.L. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology 2013, 80, 1734–1739. [Google Scholar] [CrossRef]
- Amezcua, L.; McCauley, J.L. Race and ethnicity on MS presentation and disease course. Mult. Scler. 2020, 26, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Hittle, M.; Culpepper, W.J.; Langer-Gould, A.; Marrie, R.A.; Cutter, G.R.; Kaye, W.E.; Wagner, L.; Topol, B.; LaRocca, N.G.; Nelson, L.M.; et al. Population-Based Estimates for the Prevalence of Multiple Sclerosis in the United States by Race, Ethnicity, Age, Sex, and Geographic Region. JAMA Neurol. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Singhal, B.S.; Advani, H. Multiple sclerosis in India: An overview. Ann. Indian Acad. Neurol. 2015, 18, S2. [Google Scholar] [CrossRef]
- Bhatia, R.; Bali, P.; Chaudhari, R.M. Epidemiology and genetic aspects of multiple sclerosis in India. Ann. Indian Acad. Neurol. 2015, 18, S6. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Alroughani, R.; Ahmed, S.F.; Behbahani, R.; Khan, R.; Thussu, A.; Alexander, K.J.; Ashkanani, A.; Nagarajan, V.; Al-Hashel, J. Increasing prevalence and incidence rates of multiple sclerosis in Kuwait. Mult. Scler. 2014, 20, 543–547, Erratum in Mult. Scler. 2017, 23, NP1. [Google Scholar] [CrossRef]
- Deleu, D.; Mir, D.; Al Tabouki, A.; Mesraoua, R.; Mesraoua, B.; Akhtar, N.; Al Hail, H.; D’souza, A.; Melikyan, G.; Imam, Y.Z.; et al. Prevalence, demographics and clinical characteristics of multiple sclerosis in Qatar. Mult. Scler. 2013, 19, 816–819. [Google Scholar] [CrossRef]
- Alsharoqi, I.; Alsaffar, M.; Almukhtar, B.; Abdulla, F.; Aljishi, A. Prevalence, demographics and clinical features of multiple sclerosis in Bahrain. Mult. Scler. Relat. Disord. 2014, 3, 761. [Google Scholar] [CrossRef]
- Inshasi, J.; Thakre, M. Prevalence of multiple sclerosis in Dubai, United Arab Emirates. Int. J. Neurosci. 2011, 121, 393–398. [Google Scholar] [CrossRef]
- Schiess, N.; Huether, K.; Fatafta, T.; Fitzgerald, K.C.; Calabresi, P.A.; Blair, I.; Alsaadi, T.; Szolics, M. How global MS prevalence is changing: A retrospective chart review in the United Arab Emirates. Mult. Scler. Relat. Disord. 2016, 9, 73–79. [Google Scholar] [CrossRef]
- Gunn, H.; Andrade, J.; Paul, L.; Miller, L.; Creanor, S.; Green, C.; Marsden, J.; Ewings, P.; Berrow, M.; Vickery, J.; et al. Balance Right in Multiple Sclerosis (BRiMS): A guided self-management programme to reduce falls and improve quality of life, balance and mobility in people with secondary progressive multiple sclerosis: A protocol for a feasibility randomised controlled trial. Pilot Feasibility Stud. 2018, 4, 26. [Google Scholar]
- Sharma, N.; Shandilya, A.; Kumar, N.; Mehan, S. Dysregulation of SIRT-1 Signaling in Multiple Sclerosis and Neuroimmune Disorders: A Systematic Review of SIRTUIN Activators as Potential Immunomodulators and their Influences on other Dysfunctions. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1845–1868. [Google Scholar] [CrossRef] [PubMed]
- Barbour, C.; Kosa, P.; Komori, M.; Tanigawa, M.; Masvekar, R.; Wu, T.; Johnson, K.; Douvaras, P.; Fossati, V.; Herbst, R.; et al. Molecular-based diagnosis of multiple sclerosis and its progressive stage. Ann. Neurol. 2017, 82, 795–812. [Google Scholar] [CrossRef] [PubMed]
- Mehan, S. Editorial: Therapeutic modulators inhibiting neuromuscular and motor neuron degeneration. Front. Neurosci. 2023, 17, 1188945. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Rocca, M.A.; Ciccarelli, O.; De Stefano, N.; Evangelou, N.; Kappos, L.; Rovira, A.; Sastre-Garriga, J.; Tintorè, M.; Frederiksen, J.L.; et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016, 15, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Singhal, A.; Bhatia, R.; Srivastava, M.P.; Prasad, K.; Singh, M.B. Multiple sclerosis in India: An institutional study. Mult. Scler. Relat. Disord. 2015, 4, 250–257. [Google Scholar] [CrossRef]
- Ford, H. Clinical presentation and diagnosis of multiple sclerosis. Clin. Med. 2020, 20, 380–383. [Google Scholar] [CrossRef]
- Deisenhammer, F.; Zetterberg, H.; Fitzner, B.; Zettl, U.K. The cerebrospinal fluid in multiple sclerosis. Front. Immunol. 2019, 10, 726. [Google Scholar] [CrossRef] [Green Version]
- Baiano, C.; Zeppieri, M. Visual Evoked Potential. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Singhal, B. Multiple sclerosis-Indian perspective. Neurol India 2015, 63, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Jena, S.S.; Aaron, S.; Mathew, V.; Thomas, M.M.; Patil, A.K.; Sivadasan, A.; Muthusamy, K.; Mani, S.; Rebekah, J.G. Natural history of multiple sclerosis from the Indian perspective: Experience from a tertiary care hospital. Neurol. India 2015, 63, 866. [Google Scholar] [CrossRef]
- Shah, P. Symptomatic management in multiple sclerosis. Ann. Indian Acad. Neurol. 2015, 18, S35–S42. [Google Scholar] [CrossRef] [PubMed]
- Burks, J.S.; Bigley, G.K.; Hill, H.H. Rehabilitation challenges in multiple sclerosis. Ann. Indian Acad. Neurol. 2009, 12, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Upadhayay, S.; Mehan, S.; Prajapati, A.; Sethi, P.; Suri, M.; Zawawi, A.; Almashjary, M.N.; Tabrez, S. Nrf2/HO-1 Signaling Stimulation through Acetyl-11-Keto-Beta-Boswellic Acid (AKBA) Provides Neuroprotection in Ethidium Bromide-Induced Experimental Model of Multiple Sclerosis. Genes 2022, 13, 1324. [Google Scholar] [CrossRef] [PubMed]
- Pandit, L. Insights into the changing perspectives of multiple sclerosis in India. Autoimmune Dis. 2011, 2011, 937586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MS International Foundation. Available online: https://www.atlasofms.org/map/global/epidemiology/number-of-people-with-ms (accessed on 14 June 2023).
- India MS MAP. Available online: https://indiamsmap.org/ (accessed on 14 June 2023).
- Soldan, S.S.; Lieberman, P.M. Epstein–Barr virus and multiple sclerosis. Nat. Rev. Microbiol. 2023, 21, 51–64. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Dehghanian, F.; Nabavizadeh, N.; Kamali, E.; Moeinifar, N.; Motovali-Bashi, M. A complete review on epigenetic biomarkers in MS. In Neuroinflammation; Academic Press: Cambridge, MA, USA, 2018; pp. 619–637. [Google Scholar]
- Fransen, N.L.; Hsiao, C.C.; van der Poel, M.; Engelenburg, H.J.; Verdaasdonk, K.; Vincenten, M.C.; Remmerswaal, E.B.M.; Kuhlmann, T.; Mason, M.R.J.; Hamann, J.; et al. Tissue-resident memory T cells invade the brain parenchyma in multiple sclerosis white matter lesions. Brain 2020, 143, 1714–1730. [Google Scholar] [CrossRef]
- Hatton, O.L.; Harris-Arnold, A.; Schaffert, S.; Krams, S.M.; Martinez, O.M. The interplay between Epstein–Barr virus and B lymphocytes: Implications for infection, immunity, and disease. Immunol. Res. 2014, 58, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Thacker, E.L.; Mirzaei, F.; Ascherio, A. Infectious mononucleosis and risk for multiple sclerosis: A meta-analysis. Ann. Neurol. 2006, 59, 499–503. [Google Scholar] [CrossRef]
- Bellucci, G.; Rinaldi, V.; Buscarinu, M.C.; Reniè, R.; Bigi, R.; Pellicciari, G.; Morena, E.; Romano, C.; Marrone, A.; Mechelli, R.; et al. Multiple sclerosis and SARS-CoV-2: Has the interplay started? Front. Immunol. 2021, 12, 755333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zuo, Y.; Jiang, L.; Peng, Y.; Huang, X.; Zuo, L. Epstein-Barr Virus and Neurological Diseases. Front. Mol. Biosci. 2022, 8, 816098. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and multiple sclerosis: A comprehensive review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munger, K.L.; Zhang, S.M.; O’reilly, E.; Hernan, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef]
- Aparna, P.; Muthathal, S.; Nongkynrih, B.; Gupta, S.K. Vitamin D deficiency in India. J. Fam. Med. Prim. Care 2018, 7, 324. [Google Scholar]
- Pandit, L.; Ramagopalan, S.V.; Malli, C.; D’Cunha, A.; Kunder, R.; Shetty, R. Association of vitamin D and multiple sclerosis in India. Mult. Scler. J. 2013, 19, 1592–1596. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munger, K.L.; Ascherio, A. Prevention and treatment of MS: Studying the effects of vitamin D. Mult. Scler. 2011, 17, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Miclea, A.; Bagnoud, M.; Chan, A.; Hoepner, R. A brief review of the effects of vitamin D on multiple sclerosis. Front. Immunol. 2020, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Gombash, S.E.; Lee, P.W.; Sawdai, E.; Lovett-Racke, A.E. Vitamin D as a risk factor for multiple sclerosis: Immunoregulatory or neuroprotective? Front. Neurol. 2022, 13, 796933. [Google Scholar] [CrossRef] [PubMed]
- Cassard, S.D.; Fitzgerald, K.C.; Qian, P.; Emrich, S.A.; Azevedo, C.J.; Goodman, A.D.; Sugar, E.A.; Pelletier, D.; Waubant, E.; Mowry, E.M. High-dose vitamin D3 supplementation in relapsing-remitting multiple sclerosis: A randomised clinical trial. EClinicalMedicine 2023, 59, 101957. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, F.M. Update in vitamin D and multiple sclerosis. Neurosciences 2015, 20, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Mitrovič, M.; Patsopoulos, N.A.; Beecham, A.H.; Dankowski, T.; Goris, A.; Dubois, B.; Cotsapas, C. Low-frequency and rare-coding variation contributes to multiple sclerosis risk. Cell 2018, 175, 1679–1687. [Google Scholar] [CrossRef] [Green Version]
- Waubant, E.; Lucas, R.; Mowry, E.; Graves, J.; Olsson, T.; Alfredsson, L.; Langer-Gould, A. Environmental and genetic risk factors for MS: An integrated review. Ann. Clin. Transl. Neurol. 2019, 6, 1905–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawcer, S.; Franklin, R.J.; Ban, M. Multiple sclerosis genetics. Lancet Neurol. 2014, 13, 700–709. [Google Scholar] [CrossRef]
- Dyment, D.A.; Sadovnick, A.D.; Ebers, G.C. Genetics of multiple sclerosis. Hum. Mol. Genet. 1997, 6, 1693–1698, Erratum in Hum. Mol. Genet. 1997, 6, 2189. [Google Scholar] [CrossRef] [PubMed]
- Briggs, F.B.; Gunzler, D.D.; Ontaneda, D.; Marrie, R.A. Smokers with MS have greater decrements in quality of life and disability than non-smokers. Mult. Scler. J. 2017, 23, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Nishanth, K.; Tariq, E.; Nzvere, F.P.; Miqdad, M.; Cancarevic, I. Role of smoking in the pathogenesis of multiple sclerosis: A review article. Cureus 2020, 12, e9564. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M. Smoking: Effects on multiple sclerosis susceptibility and disease progression. Ther. Adv. Neurol. Disord. 2012, 5, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Gorman, C.; Lucas, R.; Taylor, B. Environmental risk factors for multiple sclerosis: A review with a focus on molecular mechanisms. Int. J. Mol. Sci. 2012, 13, 11718–11752. [Google Scholar] [CrossRef] [Green Version]
- Alrouji, M.; Manouchehrinia, A.; Gran, B.; Constantinescu, C.S. Effects of cigarette smoke on immunity, neuroinflammation and multiple sclerosis. J. Neuroimmunol. 2019, 329, 24–34. [Google Scholar] [CrossRef]
- Baskara, I.; Kerbrat, S.; Dagouassat, M.; Nguyen, H.Q.; Guillot-Delost, M.; Surenaud, M.; Baillou, C.; Lemoine, F.M.; Morin, D.; Boczkowski, J.; et al. Cigarette smoking induces human CCR6+ Th17 lymphocytes senescence and VEGF-A secretion. Sci. Rep. 2020, 10, 6488. [Google Scholar] [CrossRef] [Green Version]
- Hedström, A.K.; Hillert, J.; Olsson, T.; Alfredsson, L. Smoking and multiple sclerosis susceptibility. Eur. J. Epidemiol. 2013, 28, 867–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abna, Z.; Fazeli, S.A.; Mirhashemi, S.; Mirzaei, K.; Emami, F.; Jamili, S.; Dehghani, R. A narrative review study on the effects of obesity and bariatric surgery on multiple sclerosis. Ann. Indian Acad. Neurol. 2021, 24, 664. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.A.; Barcellos, L.F. Obesity and multiple sclerosis susceptibility: A review. J. Neurol. Neuromed. 2016, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Balasa, R.; Maier, S.; Barcutean, L.; Stoian, A.; Motataianu, A. The direct deleterious effect of Th17 cells in the nervous system compartment in multiple sclerosis and experimental autoimmune encephalomyelitis: One possible link between neuroinflammation and neurodegeneration. Rev. Romana Med. Lab. 2020, 28, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Raud, B.; McGuire, P.J.; Jones, R.G.; Sparwasser, T.; Berod, L. Fatty acid metabolism in CD8+ T cell memory: Challenging current concepts. Immunol. Rev. 2018, 283, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Harbo, H.F.; Gold, R.; Tintoré, M. Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Sellner, J.; Kraus, J.; Awad, A.; Milo, R.; Hemmer, B.; Stüve, O. The increasing incidence and prevalence of female multiple sclerosis—A critical analysis of potential environmental factors. Autoimmun. Rev. 2011, 10, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, C.; Le Page, E.; Kantarci, O.; Siva, A.; Pelletier, D.; Okuda, D.T.; Club Francophone de la Scleroseen Plaques (CFSEP) and the Radiologically Isolated Syndrome Consortium (RISC) Group. Impact of pregnancy on conversion to clinically isolated syndrome in a radiologically isolated syndrome cohort. Mult. Scler. J. 2012, 18, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Bove, R.; Chitnis, T. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult. Scler. J. 2014, 20, 520–526. [Google Scholar] [CrossRef] [PubMed]
- WitmanTsur, S.; Adrian Zaher, E.; Tsur, M.; Kania, K.; Kalinowska-Łyszczarz, A. Current immunological and clinical perspective on vaccinations in multiple sclerosis patients: Are they safe after all? Int. J. Mol. Sci. 2021, 22, 3859. [Google Scholar] [CrossRef]
- Dargahi, N.; Katsara, M.; Tselios, T.; Androutsou, M.E.; De Courten, M.; Matsoukas, J.; Apostolopoulos, V. Multiple sclerosis: Immunopathology and treatment update. Brain Sci. 2017, 7, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petermann, F.; Korn, T. Cytokines and effector T cell subsets causing autoimmune CNS disease. FEBS Lett. 2011, 585, 3747–3757. [Google Scholar] [CrossRef] [Green Version]
- Lock, C.; Hermans, G.; Pedotti, R.; Brendolan, A.; Schadt, E.; Garren, H.; Langer-Gould, A.; Strober, S.; Cannella, B.; Allard, J.; et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002, 8, 500–508. [Google Scholar] [CrossRef]
- Kebir, H.; Ifergan, I.; Alvarez, J.I.; Bernard, M.; Poirier, J.; Arbour, N.; Duquette, P.; Prat, A. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann. Neurol. 2009, 66, 390–402. [Google Scholar] [CrossRef]
- Balashov, K.E.; Rottman, J.B.; Weiner, H.L.; Hancock, W.W. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc. Natl. Acad. Sci. USA 1999, 96, 6873–6878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzartos, J.S.; Friese, M.A.; Craner, M.J.; Palace, J.; Newcombe, J.; Esiri, M.M.; Fugger, L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008, 172, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Panitch, H.S.; Hirsch, R.L.; Haley, A.S.; Johnson, K.P. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet 1987, 1, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, W.J.; Solomon, A.J. Misdiagnosis of multiple sclerosis: Time for action. Mult. Scler. J. 2021, 27, 805–806. [Google Scholar] [CrossRef] [PubMed]
- MS International Federation. Atlas of MS. 2013. Available online: https://www.msif.org/about-us/advocacy/atlas/ (accessed on 12 September 2016).
- Kazmierski, R.; Wierzbicka, M.; Kotecka-Sowinska, E.; Banaszewski, J.; Pawlak, M.A. Expansion of the Classification System for Eagle Syndrome. Ann. Intern. Med. 2018, 168, 746–747. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, S.S. An Unusually Long Process: Eagle Syndrome. Am. J. Med. 2016, 129, 1166–1167. [Google Scholar] [CrossRef] [Green Version]
- Morrison, R.J.; Morrison, P.J. Aetiology of Eagle syndrome: Ossification of the stylohyoid ligament. QJM 2019, 112, 467. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, T.; Akgün, K.; Brück, W. Molecular biomarkers in multiple sclerosis. J. Neuroinflamm. 2019, 16, 272. [Google Scholar] [CrossRef] [Green Version]
- Phuljhele, S.; Kedar, S.; Saxena, R. Approach to optic neuritis: An update. Indian J. Ophthalmol. 2021, 69, 2266. [Google Scholar]
- Pandit, L.; Sato, D.K.; Mustafa, S.; Takahashi, T.; D’Cunha, A.; Malli, C.; Sudhir, A.; Fujihara, K. Serological markers associated with neuromyelitis optica spectrum disorders in South India. Ann. Indian Acad. Neurol. 2016, 19, 505. [Google Scholar] [CrossRef]
- Ross, A.P.; Hackbarth, N.; Rohl, C.; Whitmyre, K. Effective multiple sclerosis management through improved patient assessment. J. Neurosci. Nurs. 2008, 40, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Pandit, L.; Malli, C.; D’Cunha, A.; Sudhir, A. Overcoming the challenges in diagnosis of AQP4-IgG positive neuromyelitis optica spectrum disorders in resource poor settings using an indigenized and cost effective cell based assay. J. Neuroimmunol. 2021, 360, 577706. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.J.; Pettigrew, R.; Naismith, R.T.; Chahin, S.; Krieger, S.; Weinshenker, B. Challenges in multiple sclerosis diagnosis: Misunderstanding and misapplication of the McDonald criteria. Mult. Scler. J. 2021, 27, 250–258. [Google Scholar] [CrossRef]
- Kaunzner, U.W.; Gauthier, S.A. MRI in the assessment and monitoring of multiple sclerosis: An update on best practice. Ther. Adv. Neurol. Disord. 2017, 10, 247–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aruru, M.V.; Salmon, J.W.; Douglas, G.W. Multiple Sclerosis in India: A qualitative study of access, adherence and other issues in Southern India. In Proceedings of the Business and Health Administration Association Annual Conference, Chicago, IL, USA, 22 March 2014. [Google Scholar]
- Boski, N.; Gulati, V.; Raj, R.; Gulati, P. Multiple Sclerosis-Minimizing Errors in Radiological Diagnosis. Neurol. India 2021, 69, 1539. [Google Scholar] [PubMed]
- Valentin, M.A.; Ma, S.; Zhao, A.; Legay, F.; Avrameas, A. Validation of immunoassay for protein biomarkers: Bioanalytical study plan implementation to support pre-clinical and clinical studies. J. Pharm. Biomed. Anal. 2011, 55, 869–877. [Google Scholar] [CrossRef]
- Paul, A.; Comabella, M.; Gandhi, R. Biomarkers in multiple sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a029058. [Google Scholar] [CrossRef]
- Rifai, N.; Gillette, M.A.; Carr, S.A. Protein biomarker discovery and validation: The long and uncertain path to clinical utility. Nat. Biotechnol. 2006, 24, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Poisson, L.M. “Omics” data and levels of evidence for biomarker discovery. Genomics 2009, 93, 13–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, K.D.; Aghaeepour, N.; Ahn, A.H.; Angst, M.S.; Borsook, D.; Brenton, A.; Burczynski, M.E.; Crean, C.; Edwards, R.; Gaudilliere, B.; et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: Challenges and opportunities. Nat. Rev. Neurol. 2020, 16, 381–400. [Google Scholar] [CrossRef]
- Miller, D.H.; Weinshenker, B.G.; Filippi, M.; Banwell, B.L.; Cohen, J.A.; Freedman, M.S.; Polman, C.H. Differential diagnosis of suspected multiple sclerosis: A consensus approach. Mult. Scler. J. 2008, 14, 1157–1174. [Google Scholar] [CrossRef]
- Hartung, D.M.; Bourdette, D.N.; Ahmed, S.M.; Whitham, R.H. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: Too big to fail? Neurology 2015, 84, 2185–2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandit, L. Fair and equitable treatment for multiple sclerosis in resource-poor regions: The need for off-label therapies and regional treatment guidelines. Mult. Scler. J. 2021, 27, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Statistics, India; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Pryce, G.; Baker, D. Oligoclonal bands in multiple sclerosis; Functional significance and therapeutic implications. Does the specificity matter? Mult. Scler. Relat. Disord. 2018, 25, 131–137. [Google Scholar] [CrossRef]
- Mathur, D.; Mishra, B.K.; Rout, S.; Lopez-Iranzo, F.J.; Lopez-Rodas, G.; Vallamkondu, J.; Kandimalla, R.; Casanova, B. Potential biomarkers associated with multiple sclerosis pathology. Int. J. Mol. Sci. 2021, 22, 10323. [Google Scholar] [CrossRef]
- Kuhle, J.; Disanto, G.; Dobson, R.; Adiutori, R.; Bianchi, L.; Topping, J.; Bestwick, J.P.; Meier, U.-C.; Marta, M.; Costa, G.D. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicentre study. Mult. Scler. J. 2015, 21, 1013–1024. [Google Scholar] [CrossRef]
- Matute-Blanch, C.; Villar, L.M.; Álvarez-Cermeño, J.C.; Rejdak, K.; Evdoshenko, E.; Makshakov, G.; Nazarov, V.; Lapin, S.; Midaglia, L.; Vidal-Jordana, A.; et al. Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain 2018, 141, 1085–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Sharma, N.; Khera, R.; Gupta, R.; Mehan, S. Guggulsterone ameliorates ethidium bromide-induced experimental model of multiple sclerosis via restoration of behavioral, molecular, neurochemical and morphological alterations in rat brain. Metab. Brain Dis. 2021, 36, 911–925. [Google Scholar] [CrossRef]
- Farina, G.; Magliozzi, R.; Pitteri, M.; Reynolds, R.; Rossi, S.; Gajofatto, A.; Benedetti, M.D.; Facchiano, F.; Monaco, S.; Calabrese, M. Increased cortical lesion load and intrathecal inflammation is associated with oligoclonal bands in multiple sclerosis patients: A combined CSF and MRI study. J. Neuroinflamm. 2017, 14, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graner, M.; Pointon, T.; Manton, S.; Green, M.; Dennison, K.; Davis, M.; Braiotta, G.; Craft, J.; Edwards, T.; Polonsky, B.; et al. Oligoclonal IgG antibodies in multiple sclerosis target patient-specific peptides. PLoS ONE 2020, 15, e0228883. [Google Scholar] [CrossRef]
- Flanagan, E.P.; Cabre, P.; Weinshenker, B.G.; Sauver, J.S.; Jacobson, D.J.; Majed, M.; Lennon, V.A.; Lucchinetti, C.F.; McKeon, A.; Matiello, M.; et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann. Neurol. 2016, 79, 775–783. [Google Scholar] [CrossRef] [PubMed]
- McCreary, M.; Mealy, M.A.; Wingerchuk, D.M.; Levy, M.; DeSena, A.; Greenberg, B.M. Updated diagnostic criteria for neuromyelitis optica spectrum disorder: Similar outcomes of previously separate cohorts. Mult. Scler. J.-Exp. Transl. Clin. 2018, 4, 2055217318815925. [Google Scholar] [CrossRef] [Green Version]
- Abdel Naseer, M.; Rabah, A.M.; Rashed, L.A.; Hassan, A.; Fouad, A.M. Glutamate and Nitric Oxide as biomarkers for disease activity in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 38, 101873. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Lassmann, H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002, 1, 232–241. [Google Scholar] [CrossRef]
- Encinas, J.M.; Manganas, L.; Enikolopov, G. Nitric oxide and multiple sclerosis. Curr. Neurol. Neurosci. Rep. 2005, 5, 232–238. [Google Scholar] [CrossRef]
- Lan, M.; Tang, X.; Zhang, J.; Yao, Z. Insights in pathogenesis of multiple sclerosis: Nitric oxide may induce mitochondrial dysfunction of oligodendrocytes. Rev. Neurosci. 2018, 29, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Abdelhak, A.; Huss, A.; Kassubek, J.; Tumani, H.; Otto, M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci. Rep. 2018, 8, 14798. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayrignac, X.; Le Bars, E.; Duflos, C.; Hirtz, C.; MaleskaMaceski, A.; Carra-Dallière, C.; Charif, M.; Pinna, F.; Prin, P.; Menjot de Champfleur, N.; et al. Serum GFAP in multiple sclerosis: Correlation with disease type and MRI markers of disease severity. Sci. Rep. 2020, 10, 10923. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, M.; Malmeström, C.; Nilsson, S.; Haghighi, S.; Rosengren, L.; Lycke, J. Glial fibrillary acidic protein: A potential biomarker for progression in multiple sclerosis. J. Neurol. 2011, 258, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, V.; Kursula, P. Multiple sclerosis and myelin basic protein: Insights into protein disorder and disease. Amino Acids 2022, 54, 99–109. [Google Scholar] [CrossRef]
- Mirzaii-Dizgah, M.H.; Mirzaii-Dizgah, M.R.; Mirzaii-Dizgah, I. Serum and saliva Myelin basic protein as Multiple sclerosis biomarker. Basic Clin. Neurosci. 2021, 12, 309. [Google Scholar] [CrossRef]
- Singh, A.; Upadhayay, S.; Mehan, S. Understanding Abnormal c-JNK/p38MAPK Signaling Overactivation Involved in the Progression of Multiple Sclerosis: Possible Therapeutic Targets and Impact on Neurodegenerative Diseases. Neurotox. Res. 2021, 39, 1630–1650. [Google Scholar] [CrossRef] [PubMed]
- Atya, H.B.; Ali, S.A.; Hegazy, M.I.; El Sharkawi, F.Z. Urinary urea, uric acid and hippuric acid as potential biomarkers in multiple sclerosis patients. Indian J. Clin. Biochem. 2018, 33, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Patassini, S.; Begley, P.; Reid, S.J.; Xu, J.; Church, S.J.; Curtis, M.; Dragunow, M.; Waldvogel, H.J.; Unwin, R.D.; Snell, R.G.; et al. Identification of elevated urea as a severe, ubiquitous metabolic defect in the brain of patients with Huntington’s disease. Biochem. Biophys. Res. Commun. 2015, 468, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Begley, P.; Church, S.J.; Patassini, S.; Hollywood, K.A.; Jüllig, M.; Curtis, M.A.; Waldvogel, H.J.; Faull, R.L.M.; Unwin, R.D.; et al. Graded perturbations of metabolism in multiple regions of human brain in Alzheimer’s disease: Snapshot of a pervasive metabolic disorder. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Pakpoor, J.; Seminog, O.O.; Ramagopalan, S.V.; Goldacre, M.J. Clinical associations between gout and multiple sclerosis, Parkinson’s disease and motor neuron disease: Record-linkage studies. BMC Neurol. 2015, 15, 16. [Google Scholar] [CrossRef] [Green Version]
- Rejdak, K.; Leary, S.M.; Petzold, A.; Thompson, A.J.; Miller, D.H.; Giovannoni, G. Urinary neopterin and nitric oxide metabolites as markers of interferon β-1a activity in primary progressive multiple sclerosis. Mult. Scler. J. 2010, 16, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Gholipour, T.; Ghazizadeh, T.; Babapour, S.; Mansouri, B.; Ghaffarpour, M.; Siroos, B.; Harirchian, M.H. Decreased urinary level of melatonin as a marker of disease severity in patients with multiple sclerosis. Iran. J. Allergy Asthma Immunol. 2015, 14, 91–97. [Google Scholar]
- Kawai, T.; Ukai, H.; Inoue, O.; Maejima, Y.; Fukui, Y.; Ohashi, F.; Okamoto, S.; Takada, S.; Sakurai, H.; Ikeda, M. Evaluation of biomarkers of occupational exposure to toluene at low levels. Int. Arch. Occup. Environ. Health 2008, 81, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Ditrio, L.E.; Burroughs, A.R.; Foureau, D.M.; Haque-Begum, S.; Kasper, L.H. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2009, 183, 6041–6050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.C.; Furlano, R.I.; Jick, S.S.; Meier, C.R. Inflammatory bowel disease and the risk of autoimmune diseases. J. Crohn’s Colitis 2016, 10, 186–193. [Google Scholar] [CrossRef]
- Herman, S.; Khoonsari, P.E.; Tolf, A.; Steinmetz, J.; Zetterberg, H.; Åkerfeldt, T.; Jakobsson, P.-J.; Larsson, A.; Spjuth, O.; Burman, J.; et al. Integration of magnetic resonance imaging and protein and metabolite CSF measurements to enable early diagnosis of secondary progressive multiple sclerosis. Theranostics 2018, 8, 4477. [Google Scholar] [CrossRef] [PubMed]
- Salvisberg, C.; Tajouri, N.; Hainard, A.; Burkhard, P.R.; Lalive, P.H.; Turck, N. Exploring the human tear fluid: D iscovery of new biomarkers in multiple sclerosis. Proteom.-Clin. Appl. 2014, 8, 185–194. [Google Scholar] [CrossRef]
- Jafari, A.; Babajani, A.; Rezaei-Tavirani, M. Multiple sclerosis biomarker discoveries by proteomics and metabolomics approaches. Biomark. Insights 2021, 16, 11772719211013352. [Google Scholar] [CrossRef]
- Coyle, P.K.; Sibony, P.; Johnson, C. Oligoclonal IgG in tears. Neurology 1987, 37, 853. [Google Scholar] [CrossRef]
- Lebrun, C.; Forzy, G.; Collongues, N.; Cohen, M.; de Seze, J.; Hautecoeur, P. Club francophone de la SEP and RISConsortium. Tear analysis as a tool to detect oligoclonal bands in radiologically isolated syndrome. Rev. Neurol. 2015, 171, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Simpson, E.L. Associations of childhood eczema severity: A US population-based study. Dermatitis 2014, 25, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rammohan, K.W. Cerebrospinal fluid in multiple sclerosis. Ann. Indian Acad. Neurol. 2009, 12, 246–253. [Google Scholar] [CrossRef]
- Tarlinton, R.E.; Martynova, E.; Rizvanov, A.A.; Khaiboullina, S.; Verma, S. Role of Viruses in the Pathogenesis of Multiple Sclerosis. Viruses 2020, 12, 643. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Simpson-Yap, S.; Taylor, B.; Blizzard, L.; Lucas, R.; Ponsonby, A.L.; Broadley, S.; van der Mei, I.; AusLong/Ausimmune Investigators Group. Markers of Epstein-Barr virus and Human Herpesvirus-6 infection and multiple sclerosis clinical progression. Mult. Scler. Relat. Disord. 2022, 59, 103561. [Google Scholar] [CrossRef] [PubMed]
- Jakhmola, S.; Sk, M.F.; Chatterjee, A.; Jain, K.; Kar, P.; Jha, H.C. A plausible contributor to multiple sclerosis; presentation of antigenic myelin protein epitopes by major histocompatibility complexes. Comput. Biol. Med. 2022, 148, 105856. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Dale, R.C. Acute Disseminated Encephalomyelitis. Acute Encephalopathy and Encephalitis in Infancy and Its Related Disorders; Elsevier: Amsterdam, The Netherlands, 2018; pp. 133–142. [Google Scholar] [CrossRef]
- Yokoyama, K.; Cossu, D.; Hoshino, Y.; Tomizawa, Y.; Momotani, E.; Hattori, N. Anti-mycobacterial antibodies in paired cerebrospinal fluid and serum samples from Japanese patients with multiple sclerosis or neuromyelitis optica spectrum disorder. J. Clin. Med. 2018, 7, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühle, J.; Leppert, D.; Petzold, A.; Regeniter, A.; Schindler, C.; Mehling, M.; Anthony, D.; Kappos, L.; Lindberg, R.L.P. Neurofilament heavy chain in CSF correlates with relapses and disability in multiple sclerosis. Neurology 2011, 76, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Hinsinger, G.; Galéotti, N.; Nabholz, N.; Urbach, S.; Rigau, V.; Demattei, C.; Lehmann, S.; Camu, W.; Labauge, P.; Castelnovo, G.; et al. Chitinase 3-like proteins as diagnostic and prognostic biomarkers of multiple sclerosis. Mult. Scler. J. 2015, 21, 1251–1261. [Google Scholar] [CrossRef]
- Cantó, E.; Espejo, C.; Costa, C.; Montalban, X.; Comabella, M. Breast regression protein-39 is not required for experimental autoimmune encephalomyelitis induction. Clin. Immunol. 2015, 160, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Bonneh-Barkay, D.; Wang, G.; Starkey, A.; Hamilton, R.L.; Wiley, C.A. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J. Neuroinflamm. 2010, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.G.; Da Silva, C.A.; Dela Cruz, C.S.; Ahangari, F.; Ma, B.; Kang, M.J.; He, C.-H.; Takyar, S.; Elias, J.A. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef] [Green Version]
- Malmeström, C.; Axelsson, M.; Lycke, J.; Zetterberg, H.; Blennow, K.; Olsson, B. CSF levels of YKL-40 are increased in MS and decrease with immunosuppressive treatment. J. Neuroimmunol. 2014, 269, 87–89. [Google Scholar] [CrossRef]
- Floro, S.; Carandini, T.; Pietroboni, A.M.; De Riz, M.A.; Scarpini, E.; Galimberti, D. Role of Chitinase 3-like 1 as a Biomarker in Multiple Sclerosis: A Systematic Review and Meta-analysis. Neurol Neuroimmunol. Neuroinflamm. 2022, 9, e1164. [Google Scholar] [CrossRef] [PubMed]
- Novakova, L.; Axelsson, M.; Khademi, M.; Zetterberg, H.; Blennow, K.; Malmeström, C.; Piehl, F.; Olsson, T.; Lycke, J. Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Mult. Scler. J. 2017, 23, 62–71. [Google Scholar] [CrossRef]
- Modvig, S.; Degn, M.; Sander, B.; Horwitz, H.; Wanscher, B.; Sellebjerg, F.; Frederiksen, J.L. Cerebrospinal fluid neurofilament light chain levels predict visual outcome after optic neuritis. Mult. Scler. J. 2016, 22, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Borràs, E.; Cantó, E.; Choi, M.; Villar, L.M.; Álvarez-Cermeño, J.C.; Chiva, C.; Montalban, X.; Vitek, O.; Comabella, M.; Sabidó, E. Protein-based classifier to predict conversion from clinically isolated syndrome to multiple sclerosis. Mol. Cell. Proteom. 2016, 15, 318–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benkert, P.; Meier, S.; Schaedelin, S.; Manouchehrinia, A.; Yaldizli, Ö.; Maceski, A.; Oechtering, J.; Achtnichts, L.; Conen, D.; Derfuss, T.; et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. Lancet Neurol. 2022, 21, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Iacobaeus, E.; Khademi, M.; Brundin, L.; Norgren, N.; Koel-Simmelink, M.; Schepens, M.; Bouwman, F.; Twaalfhoven, H.A.; Blom, H.J.; et al. Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis. Neurology 2009, 72, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Koel-Simmelink, M.J.; Teunissen, C.E.; Behradkia, P.; Blankenstein, M.A.; Petzold, A. The neurofilament light chain is not stable in vitro. Ann. Neurol. 2011, 69, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Khalil, M. Neurofilaments as biomarkers in multiple sclerosis. Mult. Scler. J. 2012, 18, 552–556. [Google Scholar] [CrossRef]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef]
- Thounaojam, M.C.; Kaushik, D.K.; Basu, A. MicroRNAs in the brain: It’s regulatory role in neuroinflammation. Mol. Neurobiol. 2013, 47, 1034–1044. [Google Scholar] [CrossRef]
- Ridolfi, E.; Fenoglio, C.; Cantoni, C.; Calvi, A.; De Riz, M.; Pietroboni, A.; Villa, C.; Serpente, M.; Bonsi, R.; Vercellino, M.; et al. Expression and genetic analysis of microRNAs involved in multiple sclerosis. Int. J. Mol. Sci. 2013, 14, 4375–4384. [Google Scholar] [CrossRef]
- Dutta, R.; Chomyk, A.M.; Chang, A.; Ribaudo, M.V.; Deckard, S.A.; Doud, M.K.; Edberg, D.D.; Bai, B.; Li, M.; Baranzini, S.E.; et al. Hippocampal demyelination and memory dysfunction are associated with increased levels of the neuronal microRNA miR-124 and reduced AMPA receptors. Ann. Neurol. 2013, 73, 637–645. [Google Scholar] [CrossRef] [PubMed]
- De Felice, B.; Mondola, P.; Sasso, A.; Orefice, G.; Bresciamorra, V.; Vacca, G.; Biffali, E.; Borra, M.; Pannone, R. Small non-coding RNA signature in multiple sclerosis patients after treatment with interferon-β. BMC Med. Genom. 2014, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Keller, A.; Leidinger, P.; Steinmeyer, F.; Stähler, C.; Franke, A.; Hemmrich-Stanisak, G.; Kappel, A.; Wright, I.; Dörr, J.; Paul, F.; et al. Comprehensive analysis of microRNA profiles in multiple sclerosis including next-generation sequencing. Mult. Scler. J. 2014, 20, 295–303. [Google Scholar] [CrossRef]
- DiSano, K.D.; Gilli, F.; Pachner, A.R. Intrathecally produced CXCL13: A predictive biomarker in multiple sclerosis. Mult. Scler. J.-Exp. Transl. Clin. 2020, 6, 2055217320981396. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.W.; Reeves, C.; Roncaroli, F.; Nicholas, R.; Serafini, B.; Aloisi, F.; Reynolds, R. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann. Neurol. 2010, 68, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Krumbholz, M.; Theil, D.; Cepok, S.; Hemmer, B.; Kivisäkk, P.; Ransohoff, R.M.; Hofbauer, M.; Farina, C.; Derfuss, T.; Hartle, C.; et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 2006, 129, 200–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, H.D.; Shelton, R.C.; Duman, R.S. Functional biomarkers of depression: Diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 2011, 36, 2375–2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, K.; Rath, A.; Schoerner, C.; Meyer, R.; Bertsch, T.; Erbguth, F.; Bogdan, C.; Steinmann, J.; Held, J. Comparative Analysis of the Euroimmun CXCL13 Enzyme-Linked Immunosorbent Assay and the ReaScan Lateral Flow Immunoassay for Diagnosis of Lyme Neuroborreliosis. J. Clin. Microbiol. 2020, 58, e00207-20. [Google Scholar] [CrossRef]
- Haglund, S.; Lager, M.; Gyllemark, P.; Andersson, G.; Ekelund, O.; Sundqvist, M.; Henningsson, A.J. CXCL13 in laboratory diagnosis of Lyme neuroborreliosis-the performance of the recomBead and ReaScan CXCL13 assays in human cerebrospinal fluid samples. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 175–179. [Google Scholar] [CrossRef]
- van der Kop, M.L.; Pearce, M.S.; Dahlgren, L.; Synnes, A.; Sadovnick, D.; Sayao, A.L.; Tremlett, H. Neonatal and delivery outcomes in women with multiple sclerosis. Ann. Neurol. 2011, 70, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pachner, A.R.; Steiner, I. Lyme neuroborreliosis: Infection, immunity, and inflammation. Lancet Neurol. 2007, 6, 544–552. [Google Scholar] [CrossRef]
- Komori, M.; Blake, A.; Greenwood, M.; Lin, Y.C.; Kosa, P.; Ghazali, D.; Winokur, P.; Natrajan, M.; Wuest, S.C.; Romm, E.; et al. Cerebrospinal fluid markers reveal intrathecal inflammation in progressive multiple sclerosis. Ann. Neurol. 2015, 78, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Michel, L.; Touil, H.; Pikor, N.B.; Gommerman, J.L.; Prat, A.; Bar-Or, A. B Cells in the Multiple Sclerosis Central Nervous System: Trafficking and Contribution to CNS-Compartmentalized Inflammation. Front. Immunol. 2015, 6, 636. [Google Scholar] [CrossRef] [Green Version]
- Chunder, R.; Schropp, V.; Kuerten, S. B Cells in Multiple Sclerosis and Virus-Induced Neuroinflammation. Front. Neurol. 2020, 11, 591894. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Keppler-Noreuil, K.M.; Parker, V.E.; Darling, T.N.; Martinez-Agosto, J.A. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies. Am. J. Med. Genet. C Semin. Med. Genet. 2016, 172, 402–421. [Google Scholar] [CrossRef] [Green Version]
- Mammana, S.; Bramanti, P.; Mazzon, E.; Cavalli, E.; Basile, M.S.; Fagone, P.; Petralia, M.C.; McCubrey, J.A.; Nicoletti, F.; Mangano, K. Preclinical evaluation of the PI3K/Akt/mTOR pathway in animal models of multiple sclerosis. Oncotarget 2018, 9, 8263–8277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrell, R.A.; Antony, D.; Wall, G.R.; Clark, D.A.; Fisniku, L.; Swanton, J.; Khaleeli, Z.; Schmierer, K.; Miller, D.H.; Giovannoni, G. Humoral immune response to EBV in multiple sclerosis is associated with disease activity on MRI. Neurology 2009, 73, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Gibson, S.A.; Buckley, J.A.; Qin, H.; Benveniste, E.N. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin. Immunol. 2018, 189, 4–13. [Google Scholar] [CrossRef]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. PPARgamma in Metabolism, Immunity, and Cancer: Unified and Diverse Mechanisms of Action. Front. Endocrinol. 2021, 12, 624112. [Google Scholar] [CrossRef]
- Ferret-Sena, V.; Capela, C.; Sena, A. Metabolic Dysfunction and Peroxisome Proliferator-Activated Receptors (PPAR) in Multiple Sclerosis. Int. J. Mol. Sci. 2018, 19, 1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dönder, A.; Özdemir, H.H. Serum YKL-40 levels in patients with multiple sclerosis. Arq. Neuro-Psiquiatr. 2021, 79, 795–798. [Google Scholar] [CrossRef]
- Khalil, M.; Pirpamer, L.; Hofer, E.; Voortman, M.M.; Barro, C.; Leppert, D.; Benkert, P.; Ropele, S.; Enzinger, C.; Fazekas, F.; et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat. Commun. 2020, 11, 812. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahman, H.S.; Selim, H.S.; Hashish, M.H.; Sultan, L.I. Epstein–Barr virus in multiple sclerosis. J. Egypt. Public Health Assoc. 2014, 89, 90–95. [Google Scholar] [CrossRef]
- Brändle, S.M.; Obermeier, B.; Senel, M.; Bruder, J.; Mentele, R.; Khademi, M.; Olsson, T.; Tumani, H.; Kristoferitsch, W.; Lottspeich, F.; et al. Distinct oligoclonal band antibodies in multiple sclerosis recognize ubiquitous self-proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 7864–7869. [Google Scholar] [CrossRef] [Green Version]
- Hümmert, M.W.; Wurster, U.; Bönig, L.; Schwenkenbecher, P.; Sühs, K.W.; Alvermann, S.; Gingele, S.; Skripuletz, T.; Stangel, M. Investigation of Oligoclonal IgG Bands in Tear Fluid of Multiple Sclerosis Patients. Front. Immunol. 2019, 10, 1110. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, H.B.; Hesse, D.; Krakauer, M.; Sørensen, P.S.; Sellebjerg, F. Differential microRNA expression in blood in multiple sclerosis. Mult. Scler. J. 2013, 19, 1849–1857. [Google Scholar] [CrossRef]
- Burks, J. MS: The treatment paradigm, a pathway to success for improved patient outcomes. J. Manag. Care Med. 2008, 12, 26–31. [Google Scholar]
- Naci, H.; Fleurence, R.; Birt, J.; Duhig, A. Economic burden of multiple sclerosis. Pharmacoeconomics 2010, 28, 363–379. [Google Scholar] [CrossRef]
- Owens, G.M.; Olvey, E.L.; Skrepnek, G.H.; Pill, M.W. Perspectives for managed care organizations on the burden of multiple sclerosis and the cost-benefits of disease-modifying therapies. J. Manag. Care Pharm. 2013, 19, S41–S53. [Google Scholar] [CrossRef]
- Palmer, L.; Abouzaid, S.; Shi, N.; Fowler, R.; Lenhart, G.; Dastani, H.; Kim, E. Impact of patient cost sharing on multiple sclerosis treatment. Am. J. Benefits 2012, 4, SP29–SP36. [Google Scholar]
- Cerqueira, J.J.; Compston, D.A.S.; Geraldes, R.; Rosa, M.M.; Schmierer, K.; Thompson, A.; Tinelli, M.; Palace, J. Time matters in multiple sclerosis: Can early treatment and long-term follow-up ensure everyone benefits from the latest advances in multiple sclerosis? J. Neurol. Neurosurg. Psychiatry 2018, 89, 844–850. [Google Scholar] [CrossRef]
- Kavaliunas, A.; Manouchehrinia, A.; Stawiarz, L.; Ramanujam, R.; Agholme, J.; Hedström, A.K.; Hillert, J. Importance of early treatment initiation in the clinical course of multiple sclerosis. Mult. Scler. J. 2017, 23, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Landfeldt, E.; Castelo-Branco, A.; Svedbom, A.; Löfroth, E.; Kavaliunas, A.; Hillert, J. The long-term impact of early treatment of multiple sclerosis on the risk of disability pension. J. Neurol. 2018, 265, 701–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavaliunas, A.; Manouchehrinia, A.; Gyllensten, H.; Alexanderson, K.; Hillert, J. Importance of early treatment decisions on future income of multiple sclerosis patients. Mult. Scler. J.-Exp. Transl. Clin. 2020, 6, 2055217320959116. [Google Scholar] [CrossRef] [PubMed]
- Trojano, M.; Ramió-Torrentà, L.; Grimaldi, L.M.; Lubetzki, C.; Schippling, S.; Evans, K.C.; Ren, Z.; Muralidharan, K.K.; Licata, S.; Gafson, A.R. A randomized study of natalizumab dosing regimens for relapsing–remitting multiple sclerosis. Mult. Scler. J. 2021, 27, 2240–2253. [Google Scholar] [CrossRef] [PubMed]
- Comi, G. Early treatment. Neurol. Sci. 2006, 27, S8–S12. [Google Scholar] [CrossRef] [PubMed]
- Flachenecker, P. Disease-modifying drugs for the early treatment of multiple sclerosis. Expert Rev. Neurother. 2004, 4, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Costello, K.; Kennedy, P.; Scanzillo, J. Recognizing nonadherence in patients with multiple sclerosis and maintaining treatment adherence in the long term. Medscape J. Med. 2008, 10, 225. [Google Scholar]
- Di Battista, G.; Bertolotto, A.; Gasperini, C.; Ghezzi, A.; Maimone, D.; Solaro, C. Multiple Sclerosis State of the Art (SMART): A qualitative and quantitative analysis of therapy’s adherence, hospital reliability’s perception, and services provided quality. Mult. Scler. Int. 2014, 2014, 752318. [Google Scholar] [CrossRef]
- Treadaway, K.; Cutter, G.; Salter, A.; Lynch, S.; Simsarian, J.; Corboy, J.; Jeffery, D.; Cohen, B.; Mankowski, K.; Guarnaccia, J.; et al. Factors that influence adherence with disease-modifying therapy in MS. J. Neurol. 2009, 256, 568–576. [Google Scholar] [CrossRef]
- Ivanova, J.I.; Bergman, R.E.; Birnbaum, H.G.; Phillips, A.L.; Stewart, M.; Meletiche, D.M. Impact of medication adherence to disease-modifying drugs on severe relapse, and direct and indirect costs among employees with multiple sclerosis in the US. J. Med. Econ. 2012, 15, 601–609. [Google Scholar] [CrossRef]
- Gajofatto, A.; Benedetti, M.D. Treatment strategies for multiple sclerosis: When to start, when to change, when to stop? World J. Clin. Cases 2015, 3, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Washington, F.; Langdon, D. Factors affecting adherence to disease-modifying therapies in multiple sclerosis: Systematic review. J. Neurol. 2022, 269, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Hamilton, J.P. Drug-induced Liver Injury. US Gastroenterol. Hepatol. Rev. 2010, 6, 73–80. [Google Scholar] [PubMed]
- Bianchi, A.; Ciccarelli, O. Daclizumab-induced encephalitis in multiple sclerosis. Mult. Scler. J. 2019, 25, 1557–1559. [Google Scholar] [CrossRef] [PubMed]
- Nadhem, O.N.; Al Janabi, M.; Omer, A.R.; Wan, B. Autoimmune hepatitis with multiple sclerosis and graves disease: Coincidence or association. Case Rep. Gastroenterol. 2014, 8, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Antonazzo, I.C.; Poluzzi, E.; Forcesi, E.; Riise, T.; Bjornevik, K.; Baldin, E.; Muratori, L.; De Ponti, F.; Raschi, E. Liver injury with drugs used for multiple sclerosis: A contemporary analysis of the FDA Adverse Event Reporting System. Mult. Scler. J. 2019, 25, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Biolato, M.; Bianco, A.; Lucchini, M.; Gasbarrini, A.; Mirabella, M.; Grieco, A. The Disease-Modifying Therapies of Relapsing-Remitting Multiple Sclerosis and Liver Injury: A Narrative Review. CNS Drugs 2021, 35, 861–880. [Google Scholar] [CrossRef]
- Kowalec, K.; Kingwell, E.; Yoshida, E.M.; Marrie, R.A.; Kremenchutzky, M.; Campbell, T.L.; Wadelius, M.; Carleton, B.; Tremlett, H. Characteristics associated with drug-induced liver injury from interferon beta in multiple sclerosis patients. Expert Opin. Drug Saf. 2014, 13, 1305–1317. [Google Scholar] [CrossRef]
- Wallin, M.T.; Culpepper, W.J.; Campbell, J.D.; Nelson, L.M.; Langer-Gould, A.; Marrie, R.A.; Cutter, G.R.; Kaye, W.E.; Wagner, L.; Tremlett, H.; et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 2020, 92, e1029–e1040. [Google Scholar] [CrossRef] [Green Version]
- Buscarinu, M.C.; Reniè, R.; Morena, E.; Romano, C.; Bellucci, G.; Marrone, A.; Bigi, R.; Salvetti, M.; Ristori, G. Late-Onset MS: Disease Course and Safety-Efficacy of DMTS. Front. Neurol. 2022, 13, 829331. [Google Scholar] [CrossRef] [PubMed]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.P.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef]
- Vaughn, C.B.; Jakimovski, D.; Kavak, K.S.; Ramanathan, M.; Benedict, R.H.; Zivadinov, R.; Weinstock-Guttman, B. Epidemiology and treatment of multiple sclerosis in elderly populations. Nat. Rev. Neurol. 2019, 15, 329–342. [Google Scholar] [CrossRef]
- Schwenkenbecher, P.; Wurster, U.; Konen, F.F.; Gingele, S.; Sühs, K.-W.; Wattjes, M.P.; Stangel, M.; Skripuletz, T. Impact of the McDonald Criteria 2017 on Early Diagnosis of Relapsing-Remitting Multiple Sclerosis. Front. Neurol. 2019, 10, 188. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, V.; Rodegher, M.; Moiola, L.; Comi, G. Late onset multiple sclerosis: Clinical characteristics, prognostic factors and differential diagnosis. Neurol. Sci. 2004, 25, s350–s355. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, F.; Baumann, C.; Epstein, J.; Kerschen, P.; Garot, T.; Mathey, G.; Debouverie, M.; on behalf of the LORSEP Group. Older age at multiple sclerosis onset is an independent factor of poor prognosis: A population-based cohort study. Neuroepidemiology 2017, 48, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Naseri, A.; Nasiri, E.; Sahraian, M.A.; Daneshvar, S.; Talebi, M. Clinical features of late-onset multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2021, 50, 102816. [Google Scholar] [CrossRef]
- Scalfari, A.; Lederer, C.; Daumer, M.; Nicholas, R.; Ebers, G.C.; Muraro, P.A. The relationship of age with the clinical phenotype in multiple sclerosis. Mult. Scler. J. 2016, 22, 1750–1758. [Google Scholar] [CrossRef]
- Rae-Grant, A.; Day, G.S.; Marrie, R.A.; Rabinstein, A.; Cree, B.A.; Gronseth, G.S.; Haboubi, M.; Halper, J.; Hosey, J.P.; Jones, D.E.; et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 777–788. [Google Scholar] [CrossRef] [Green Version]
- Schweitzer, F.; Laurent, S.; Fink, G.R.; Barnett, M.H.; Reddel, S.; Hartung, H.P.; Warnke, C. Age and the risks of high-efficacy disease modifying drugs in multiple sclerosis. Curr. Opin. Neurol. 2019, 32, 305–312. [Google Scholar] [CrossRef] [PubMed]
- McGinley, M.P.; Cola, P.A.; Fox, R.J.; Cohen, J.A.; Corboy, J.J.; Miller, D. Perspectives of individuals with multiple sclerosis on discontinuation of disease-modifying therapies. Mult. Scler. J. 2020, 26, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Hutchinson, M.; Hours, M.M.; Cortinovis-Tourniaire, P.; Moreau, T.; Pregnancy in Multiple Sclerosis Group. Rate of pregnancy-related relapse in multiple sclerosis. N. Engl. J. Med. 1998, 339, 285–291. [Google Scholar] [CrossRef]
- Krysko, K.M.; Bove, R.; Dobson, R.; Jokubaitis, V.; Hellwig, K. Treatment of women with multiple sclerosis planning pregnancy. Curr. Treat. Opt. Neurol. 2021, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Alroughani, R.; Alowayesh, M.S.; Ahmed, S.F.; Behbehani, R.; Al-Hashel, J. Relapse occurrence in women with multiple sclerosis during pregnancy in the new treatment era. Neurology 2018, 90, e840–e846. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.L.; Havrdova, E.K.; Horakova, D.; Izquierdo, G.; Kalincik, T.; van der Walt, A.; Terzi, M.; Alroughani, R.; Duquette, P.; Girard, M.; et al. Incidence of pregnancy and disease-modifying therapy exposure trends in women with multiple sclerosis: A contemporary cohort study. Mult. Scler. Relat. Disord. 2019, 28, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbstritt, S.; Langer-Gould, A.; Rockhoff, M.; Haghikia, A.; Queisser-Wahrendorf, A.; Gold, R.; Hellwig, K. Glatiramer acetate during early pregnancy: A prospective cohort study. Mult. Scler. J. 2016, 22, 810–816. [Google Scholar] [CrossRef]
- Thiel, S.; Langer-Gould, A.; Rockhoff, M.; Haghikia, A.; Queisser-Wahrendorf, A.; Gold, R.; Hellwig, K. Interferon-beta exposure during first trimester is safe in women with multiple sclerosis—A prospective cohort study from the German Multiple Sclerosis and Pregnancy Registry. Mult. Scler. J. 2016, 22, 801–809. [Google Scholar] [CrossRef]
- Sandberg-Wollheim, M.; Neudorfer, O.; Grinspan, A.; Weinstock-Guttman, B.; Haas, J.; Izquierdo, G.; Coyle, P.K. Pregnancy outcomes from the branded glatiramer acetate pregnancy database. Int. J. MS Care 2018, 20, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groves, A.; Kihara, Y.; Chun, J. Fingolimod: Direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J. Neurol. Sci. 2013, 328, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappos, L.; Radue, E.-W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, M.; Manshadi, S.; Naderi, N.; Dehghan, A.; Azizi, S. Unusual side effects of interferon Beta-1a in patient with multiple sclerosis. Mater. Socio-Med. 2012, 24, 203. [Google Scholar] [CrossRef] [Green Version]
- Jankovic, S.M. Injectable interferon beta-1b for the treatment of relapsing forms of multiple sclerosis. J. Inflamm. Res. 2010, 3, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappos, L.; Traboulsee, A.; Constantinescu, C.; Erälinna, J.P.; Forrestal, F.; Jongen, P.; Pollard, J.; Sandberg-Wollheim, M.; Sindic, C.; Stubinski, B.; et al. Long-term subcutaneous interferon beta-1a therapy in patients with relapsing-remitting MS. Neurology 2006, 67, 944–953. [Google Scholar] [CrossRef]
- Madsen, C. The innovative development in interferon beta treatments of relapsing-remitting multiple sclerosis. Brain Behav. 2017, 7, e00696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipi, M.; Jack, S. Interferons in the Treatment of Multiple Sclerosis: A Clinical Efficacy, Safety, and Tolerability Update. Int. J. MS Care 2020, 22, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Zadeh, A.R.; Askari, M.; Azadani, N.N.; Ataei, A.; Ghadimi, K.; Tavoosi, N.; Falahatian, M. Mechanism and adverse effects of multiple sclerosis drugs: A review article. Part 1. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 95. [Google Scholar]
- Guarnera, C.; Bramanti, P.; Mazzon, E. Alemtuzumab: A review of efficacy and risks in the treatment of relapsing remitting multiple sclerosis. Ther. Clin. Risk Manag. 2017, 13, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, Ó. Alemtuzumab in the treatment of multiple sclerosis. J. Inflamm. Res. 2014, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Cada, D.J.; Levien, T.L.; Baker, D.E. Dimethyl fumarate. Hosp. Pharm. 2013, 48, 668–679. [Google Scholar] [CrossRef] [Green Version]
- Mirabella, M.; Annovazzi, P.; Brownlee, W.; Cohen, J.A.; Kleinschnitz, C.; Wolf, C. Treatment challenges in multiple sclerosis–a continued role for glatiramer acetate? Front. Neurol. 2022, 13, 844873. [Google Scholar] [CrossRef] [PubMed]
- Caporro, M.; Disanto, G.; Gobbi, C.; Zecca, C. Two decades of subcutaneous glatiramer acetate injection: Current role of the standard dose, and new high-dose low-frequency glatiramer acetate in relapsing–remitting multiple sclerosis treatment. Patient Prefer. Adherence 2014, 8, 1123. [Google Scholar]
- Cornblath, D.R.; Bienen, E.J.; Blight, A.R. The safety profile of dalfampridine extended release in multiple sclerosis clinical trials. Clin. Ther. 2012, 34, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Tian, X.; Li, R.; Chen, C.; Li, M.; Ma, L.; Wei, R.; Zhou, Y.; Cui, Y. Dalfampridine in the treatment of multiple sclerosis: A meta-analysis of randomised controlled trials. Orphanet J. Rare Dis. 2021, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Danesi, R.; Derfuss, T.; Duddy, M.; Gallo, P.; Gold, R.; Havrdová, E.K.; Kornek, B.; Saccà, F.; Tintoré, M.; et al. Early and unrestricted access to high-efficacy disease-modifying therapies: A consensus to optimize benefits for people living with multiple sclerosis. J. Neurol. 2022, 269, 1670–1677. [Google Scholar] [CrossRef]
- Miller, A.E. Teriflunomide in multiple sclerosis: An update. Neurodegener. Dis. Manag. 2017, 7, 9–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, A.; de Seze, J.; Comabella, M. Teriflunomide in patients with relapsing–remitting forms of multiple sclerosis. CNS Drugs 2016, 30, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, A.D.; Anadani, N.; Gerwitz, L. Siponimod in the treatment of multiple sclerosis. Expert Opin. Investig. Drugs 2019, 28, 1051–1057. [Google Scholar] [CrossRef]
- Sabsabi, S.; Mikhael, E.; Jalkh, G.; Macaron, G.; Rensel, M. Clinical Evaluation of Siponimod for the Treatment of Secondary Progressive Multiple Sclerosis: Pathophysiology, Efficacy, Safety, Patient Acceptability and Adherence. Patient Prefer. Adherence 2022, 16, 1307. [Google Scholar] [CrossRef]
- Salzer, J.; Svenningsson, R.; Alping, P.; Novakova, L.; Björck, A.; Fink, K.; Islam-Jakobsson, P.; Malmeström, C.; Axelsson, M.; Vågberg, M.; et al. Rituximab in multiple sclerosis: A retrospective observational study on safety and efficacy. Neurology 2016, 87, 2074–2081. [Google Scholar] [CrossRef] [Green Version]
- Chisari, C.G.; Sgarlata, E.; Arena, S.; Toscano, S.; Luca, M.; Patti, F. Rituximab for the treatment of multiple sclerosis: A review. J. Neurol. 2022, 269, 159–183. [Google Scholar] [CrossRef]
- Atlas of MS. Available online: https://www.msif.org/wp-content/uploads/2020/12/Atlas-3rd-Edition-Epidemiology-report-EN-updated-30-9-20.pdf (accessed on 14 June 2023).
- Massacesi, L.; Parigi, A.; Barilaro, A.; Repice, A.M.; Pellicanò, G.; Konze, A.; Siracusa, G.; Taiuti, R.; Amaducci, L. Efficacy of Azathioprine on Multiple Sclerosis New Brain Lesions Evaluated Using Magnetic Resonance Imaging. Arch. Neurol. 2005, 62, 1843–1847. [Google Scholar] [CrossRef] [Green Version]
- Myhr, K.M.; Mellgren, S.I. Corticosteroids in the treatment of multiple sclerosis. Acta Neurol. Scand Suppl. 2009, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Cladribine Tablets: A Review in Relapsing MS. CNS Drugs 2018, 32, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Leist, T.; Comi, G.; Montalban, X.; Giovannoni, G.; Nolting, A.; Hicking, C.; Galazka, A.; Sylvester, E. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: An integrated analysis. Mult. Scler. Relat. Disord. 2019, 29, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Toghi, M.; Bitarafan, S. Simvastatin Therapy in Multiple Sclerosis Patients with Respect to Gut Microbiome-Friend or Foe? J. Neuroimmune Pharmacol. 2019, 14, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Binks, S.; Nicholas, J.M.; Frost, C.; Cardoso, M.J.; Ourselin, S.; Wilkie, D.; Nicholas, R.; Chataway, J. Effect of high-dose simvastatin on cognitive, neuropsychiatric, and health-related quality-of-life measures in secondary progressive multiple sclerosis: Secondary analyses from the MS-STAT randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 591–600. [Google Scholar] [CrossRef] [Green Version]
- Peyro-Saint-Paul, L.; Creveuil, C.; Heinzlef, O.; de Seze, J.; Vermersch, P.; Castelnovo, G.; Cabre, P.; Debouverie, M.; Brochet, B.; Dupuy, B.; et al. Efficacy and safety profile of memantine in patients with cognitive impairment in multiple sclerosis: A randomized, placebo-controlled study. J. Neurol. Sci. 2016, 363, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Lovera, J.F.; Frohman, E.; Brown, T.R.; Bandari, D.; Nguyen, L.; Yadav, V.; Stuve, O.; Karman, J.; Bogardus, K.; Heimburger, G.; et al. Memantine for cognitive impairment in multiple sclerosis: A randomized placebo-controlled trial. Mult. Scler. J. 2010, 16, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Krupp, L.B.; Christodoulou, C.; Melville, P.; Scherl, W.F.; MacAllister, W.S.; Elkins, L.E. Donepezil improved memory in multiple sclerosis in a randomized clinical trial. Neurology 2004, 63, 1579–1585. [Google Scholar] [CrossRef]
- Shahpouri, M.M.; Barekatain, M.; Tavakoli, M.; Badihian, S.; Shaygannejad, V. Effect of Donepezil on Cognitive Impairment, Quality of Life, and Depression in Multiple Sclerosis Patients: A Randomized Clinical Trial. Int. J. Prev. Med. 2020, 11, 69. [Google Scholar] [CrossRef]
- Sammaraiee, Y.; Yardley, M.; Keenan, L.; Buchanan, K.; Stevenson, V.; Farrell, R. Intrathecal baclofen for multiple sclerosis related spasticity: A twenty year experience. Mult. Scler. Relat. Disord. 2019, 27, 95–100. [Google Scholar] [CrossRef]
- Fox, E.; Lovett-Racke, A.E.; Gormley, M.; Liu, Y.; Petracca, M.; Cocozza, S.; Shubin, R.; Wray, S.; Weiss, M.S.; Bosco, J.A.; et al. A phase 2 multicenter study of ublituximab, a novel glycoengineered anti-CD20 monoclonal antibody, in patients with relapsing forms of multiple sclerosis. Mult. Scler. 2021, 27, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, S. Ponesimod for the treatment of relapsing multiple sclerosis. Prescriber 2022, 33, 29–30. [Google Scholar] [CrossRef]
- Ruggieri, S.; Quartuccio, M.E.; Prosperini, L. Ponesimod in the Treatment of Relapsing Forms of Multiple Sclerosis: An Update on the Emerging Clinical Data. Degener. Neurol. Neuromuscul. Dis. 2022, 12, 61–73. [Google Scholar] [CrossRef]
- Kang, C.; Blair, H.A. Ofatumumab: A Review in Relapsing Forms of Multiple Sclerosis. Drugs 2022, 82, 55–62. [Google Scholar] [CrossRef]
- Wynn, D.; Lategan, T.W.; Sprague, T.N.; Rousseau, F.S.; Fox, E.J. Monomethyl fumarate has better gastrointestinal tolerability profile compared with dimethyl fumarate. Mult. Scler. Relat. Disord. 2020, 45, 102335. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.A.; Sottosanti, E.R.; Winnick, A.; Izygon, J.; Berardino, K.; Cornett, E.M.; Kaye, A.D.; Varrassi, G.; Viswanath, O.; Urits, I. Monomethyl Fumarate (MMF, Bafiertam) for the Treatment of Relapsing Forms of Multiple Sclerosis (MS). Neurol. Int. 2021, 13, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Thöne, J.; Linker, R.A. Laquinimod in the treatment of multiple sclerosis: A review of the data so far. Drug Des. Dev. Ther. 2016, 10, 1111–1118. [Google Scholar] [CrossRef] [Green Version]
- Masek, M.; Vaneckova, M.; Krasensky, J.; Danes, J.; Havrdova, E.; Hrebikova, T.; Seidl, Z. Secondary-progressive form of multiple sclerosis: MRI changes versus clinical status. Neuroendocrinol. Lett. 2008, 29, 461. [Google Scholar]
- Freiha, J.; Riachi, N.; Chalah, M.A.; Zoghaib, R.; Ayache, S.S.; Ahdab, R. Paroxysmal symptoms in multiple sclerosis—A review of the literature. J. Clin. Med. 2020, 9, 3100. [Google Scholar] [CrossRef] [PubMed]
- Bsteh, G.; Ehling, R.; Walchhofer, L.-M.; Hegen, H.; Auer, M.; Wurth, S.; Di Pauli, F.; Wagner, M.; Reindl, M.; Deisenhammer, F.; et al. Paroxysmal and unusual symptoms as first clinical manifestation of multiple sclerosis do not indicate benign prognosis—The PaSiMS II study. PLoS ONE 2017, 12, e0181458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, Z.; Gupta, G.D.; Mehan, S. Cellular and Molecular Evidence of Multiple Sclerosis Diagnosis and Treatment Challenges. J. Clin. Med. 2023, 12, 4274. https://doi.org/10.3390/jcm12134274
Khan Z, Gupta GD, Mehan S. Cellular and Molecular Evidence of Multiple Sclerosis Diagnosis and Treatment Challenges. Journal of Clinical Medicine. 2023; 12(13):4274. https://doi.org/10.3390/jcm12134274
Chicago/Turabian StyleKhan, Zuber, Ghanshyam Das Gupta, and Sidharth Mehan. 2023. "Cellular and Molecular Evidence of Multiple Sclerosis Diagnosis and Treatment Challenges" Journal of Clinical Medicine 12, no. 13: 4274. https://doi.org/10.3390/jcm12134274
APA StyleKhan, Z., Gupta, G. D., & Mehan, S. (2023). Cellular and Molecular Evidence of Multiple Sclerosis Diagnosis and Treatment Challenges. Journal of Clinical Medicine, 12(13), 4274. https://doi.org/10.3390/jcm12134274