Association between Menopause, Postmenopausal Hormone Therapy and Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Variables
2.3. Premenopausal, Postmenopausal, Type of Menopause, and Postmenopausal Hormone Therapy Assessments
2.4. Definition and Assessment of Metabolic Syndrome
2.5. Statistical Analyses
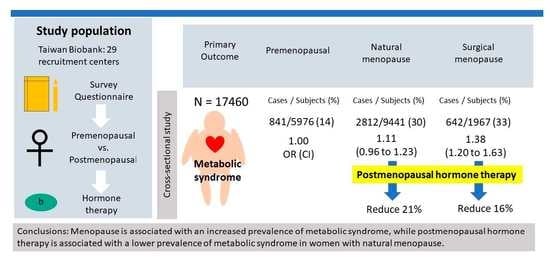
3. Results
3.1. Clinical Characteristics of the Study Participants
3.2. Associations between Menopause, Metabolic Syndrome, and Its Components
3.3. Associations between the Types of Menopause and Metabolic Syndrome
3.4. Association between Postmenopausal Hormone Therapy and Metabolic Syndrome in the Postmenopausal Women
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiBello, J.R.; Ioannou, C.; Rees, J.; Challacombe, B.; Maskell, J.; Choudhury, N.; Kastner, C.; Kirby, M. Prevalence of metabolic syndrome and its components among men with and without clinical benign prostatic hyperplasia: A large, cross-sectional, UK epidemiological study. BJU Int. 2016, 117, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B. Epidemiology of the metabolic syndrome, 2002. Am. J. Manag. Care 2002, 8 (Suppl. S11), S283–S292; quiz S293–S296. [Google Scholar] [PubMed]
- Balkau, B.; Vernay, M.; Mhamdi, L.; Novak, M.; Arondel, D.; Vol, S.; Tichet, J.; Eschwège, E. The incidence and persistence of the NCEP (National Cholesterol Education Program) metabolic syndrome. The French D.E.S.I.R. study. Diabetes Metab. 2003, 29, 526–532. [Google Scholar] [CrossRef]
- Ramachandran, A.; Snehalatha, C.; Satyavani, K.; Sivasankari, S.; Vijay, V. Metabolic syndrome in urban Asian Indian adults—A population study using modified ATP III criteria. Diabetes Res. Clin. Pract. 2003, 60, 199–204. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Chen, C.H.; Chou, P. Prevalence of metabolic syndrome in a large health check-up population in Taiwan. J. Chin. Med. Assoc. JCMA 2004, 67, 611–620. [Google Scholar]
- Lin, C.H.; Lai, S.W.; Liu, C.S. Prevalence of metabolic syndrome in Taiwanese adults: A hospital-based study. Ann. Saudi Med. 2006, 26, 46–48. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.W.; Zhu, S.; Palaniappan, L.; Heshka, S.; Carnethon, M.R.; Heymsfield, S.B. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch. Intern. Med. 2003, 163, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Miranda, P.J.; DeFronzo, R.A.; Califf, R.M.; Guyton, J.R. Metabolic syndrome: Definition, pathophysiology, and mechanisms. Am. Heart J. 2005, 149, 33–45. [Google Scholar] [CrossRef]
- Ford, E.S. Prevalence of the metabolic syndrome in US populations. Endocrinol. Metab. Clin. Nor. Am. 2004, 33, 333–350. [Google Scholar] [CrossRef]
- Kraja, A.T.; Rao, D.C.; Weder, A.B.; Mosley, T.H.; Turner, S.T.; Hsiung, C.A.; Quertermous, T.; Cooper, R.; Curb, J.D.; Province, M.A. An evaluation of the metabolic syndrome in a large multi-ethnic study: The Family Blood Pressure Program. Nutr. Metab. 2005, 2, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marjani, A.; Moghasemi, S. The Metabolic Syndrome among Postmenopausal Women in Gorgan. Int. J. Endocrinol. 2012, 2012, 953627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipsy, R.J. The National Cholesterol Education Program Adult Treatment Panel III guidelines. J. Manag. Care Pharm. JMCP 2003, 9 (Suppl. S1), 2–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurka, M.J.; Vishnu, A.; Santen, R.J.; DeBoer, M.D. Progression of Metabolic Syndrome Severity During the Menopausal Transition. J. Am. Heart Assoc. 2016, 5, 3609. [Google Scholar] [CrossRef] [Green Version]
- Pu, D.; Tan, R.; Yu, Q.; Wu, J. Metabolic syndrome in menopause and associated factors: A meta-analysis. Climacteric J. Int. Menopause Soc. 2017, 20, 583–591. [Google Scholar] [CrossRef]
- Baber, R.J.; Panay, N.; Fenton, A. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric J. Int. Menopause Soc. 2016, 19, 109–150. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Chen, J.L.; Luo, Y.; Mathur, M.B.; Anagnostis, P.; Nurmatov, U.; Talibov, M.; Zhang, J.; Hawrylowicz, C.M.; Lumsden, M.A.; et al. Menopausal hormone therapy and women’s health: An umbrella review. PLoS Med. 2021, 18, e1003731. [Google Scholar] [CrossRef]
- Coyoy, A.; Guerra-Araiza, C.; Camacho-Arroyo, I. Metabolism Regulation by Estrogens and Their Receptors in the Central Nervous System Before and After Menopause. Horm. Metab. Res. 2016, 48, 489–496. [Google Scholar] [CrossRef]
- Morselli, E.; Santos, R.S.; Criollo, A.; Nelson, M.D.; Palmer, B.F.; Clegg, D.J. The effects of oestrogens and their receptors on cardiometabolic health. Nat. Rev. Endocrinol. 2017, 13, 352–364. [Google Scholar] [CrossRef]
- Chang, C.W.; Ke, H.L.; Lee, J.I.; Lee, Y.C.; Jhan, J.H.; Wang, H.S.; Shen, J.T.; Tsao, Y.H.; Huang, S.P.; Geng, J.H. Metabolic Syndrome Increases the Risk of Kidney Stone Disease: A Cross-Sectional and Longitudinal Cohort Study. J. Pers. Med. 2021, 11, 1154. [Google Scholar] [CrossRef]
- Tang, T.Y.; Lee, J.I.; Shen, J.T.; Lee, Y.C.; Wang, H.S.; Tsao, Y.H.; Wu, Y.H.; Huang, S.P.; Chen, S.C.; Jhan, J.H.; et al. The association between menopause, postmenopausal hormone therapy, and kidney stone disease in Taiwanese women. Ann. Epidemiol. 2023, 78, 13–18. [Google Scholar] [CrossRef]
- Chen, C.H.; Lee, J.I.; Jhan, J.H.; Lee, Y.C.; Geng, J.H.; Chen, S.C.; Hung, C.H.; Kuo, C.H. Secondhand smoke increases the risk of developing kidney stone disease. Sci. Rep. 2021, 11, 17694. [Google Scholar] [CrossRef]
- Stephens, C.R.; Easton, J.F.; Robles-Cabrera, A.; Fossion, R.; de la Cruz, L.; Martínez-Tapia, R.; Barajas-Martínez, A.; Hernández-Chávez, A.; López-Rivera, J.A.; Rivera, A.L. The Impact of Education and Age on Metabolic Disorders. Front. Public Health 2020, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Damiri, B.; Khatib, O.; Nazzal, Z.; Sanduka, D.; Igbaria, S.; Thabaleh, A.; Farhoud, A.; Saudi, L.; Belkebir, S.; Al Ali, R.; et al. Metabolic Syndrome Associated with Tobacco and Caffeine Products Use Among Refugee Adolescents: Risk of Dyslipidemia. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 4121–4133. [Google Scholar] [CrossRef] [PubMed]
- Corbi-Cobo-Losey, M.J.; Martinez-Gonzalez, M.; Gribble, A.K.; Fernandez-Montero, A.; Navarro, A.M.; Domínguez, L.J.; Bes-Rastrollo, M.; Toledo, E. Coffee Consumption and the Risk of Metabolic Syndrome in the ‘Seguimiento Universidad de Navarra’ Project. Antioxidants 2023, 12, 686. [Google Scholar] [CrossRef]
- Hernández-Rubio, A.; Sanvisens, A.; Bolao, F.; Cachón-Suárez, I.; Garcia-Martín, C.; Short, A.; Bataller, R.; Muga, R. Prevalence and associations of metabolic syndrome in patients with alcohol use disorder. Sci. Rep. 2022, 12, 2625. [Google Scholar] [CrossRef]
- Méndez-Hernández, P.; Flores, Y.; Siani, C.; Lamure, M.; Dosamantes-Carrasco, L.D.; Halley-Castillo, E.; Huitrón, G.; Talavera, J.O.; Gallegos-Carrillo, K.; Salmerón, J. Physical activity and risk of metabolic syndrome in an urban Mexican cohort. BMC Public Health 2009, 9, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.A.; Kang, L.L.; Kim, H.N.; Park, H.K.; Hwang, H.S.; Park, K.Y. Relationship between Marital Status and Metabolic Syndrome in Korean Middle-Aged Women: The Sixth Korea National Health and Nutrition Examination Survey (2013–2014). Korean J. Fam. Med. 2018, 39, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Akter, S.; Jesmin, S.; Rahman, M.M.; Islam, M.M.; Khatun, M.T.; Yamaguchi, N.; Akashi, H.; Mizutani, T. Higher gravidity and parity are associated with increased prevalence of metabolic syndrome among rural Bangladeshi women. PLoS ONE 2013, 8, e68319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuebe, A.M. Does breastfeeding prevent the metabolic syndrome, or does the metabolic syndrome prevent breastfeeding? Semin. Perinatol. 2015, 39, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Lovre, D.; Lindsey, S.H.; Mauvais-Jarvis, F. Effect of menopausal hormone therapy on components of the metabolic syndrome. Ther. Adv. Cardiovasc. Dis. 2016, 11, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, G.V. Metabolic syndrome and chronic kidney disease: Current status and future directions. World J. Nephrol. 2014, 3, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Tandon, V.R.; Mahajan, A.; Sharma, S.; Sharma, A. Prevalence of cardiovascular risk factors in postmenopausal women: A rural study. J. Mid-Life Health 2010, 1, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Ainy, E.; Mirmiran, P.; Zahedi Asl, S.; Azizi, F. Prevalence of metabolic syndrome during menopausal transition Tehranian women: Tehran Lipid and Glucose Study (TLGS). Maturitas 2007, 58, 150–155. [Google Scholar] [CrossRef]
- Hidalgo, L.A.; Chedraui, P.A.; Morocho, N.; Alvarado, M.; Chavez, D.; Huc, A. The metabolic syndrome among postmenopausal women in Ecuador. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2006, 22, 447–454. [Google Scholar] [CrossRef]
- Piché, M.E.; Weisnagel, S.J.; Corneau, L.; Nadeau, A.; Bergeron, J.; Lemieux, S. The WHO and NCEP/ATPIII Definitions of the Metabolic Syndrome in Postmenopausal Women: Are They So Different? Metab. Syndr. Relat. Disord. 2006, 4, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Deibert, P.; König, D.; Vitolins, M.Z.; Landmann, U.; Frey, I.; Zahradnik, H.P.; Berg, A. Effect of a weight loss intervention on anthropometric measures and metabolic risk factors in pre- versus postmenopausal women. Nutr. J. 2007, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.M.; Kim, D.J.; Jung, I.H.; Park, C.; Park, J. Prevalence of the metabolic syndrome among Korean adults using the new International Diabetes Federation definition and the new abdominal obesity criteria for the Korean people. Diabetes Res. Clin. Pract. 2007, 77, 99–106. [Google Scholar] [CrossRef]
- Janssen, I.; Powell, L.H.; Crawford, S.; Lasley, B.; Sutton-Tyrrell, K. Menopause and the metabolic syndrome: The Study of Women’s Health Across the Nation. Arch. Intern. Med. 2008, 168, 1568–1575. [Google Scholar] [CrossRef] [Green Version]
- Petri Nahas, E.A.; Padoani, N.P.; Nahas-Neto, J.; Orsatti, F.L.; Tardivo, A.P.; Dias, R. Metabolic syndrome and its associated risk factors in Brazilian postmenopausal women. Climacteric J. Int. Menopause Soc. 2009, 12, 431–438. [Google Scholar] [CrossRef]
- Ebrahimpour, P.; Fakhrzadeh, H.; Heshmat, R.; Ghodsi, M.; Bandarian, F.; Larijani, B. Metabolic syndrome and menopause: A population-based study. Diabetes Metab. Syndr. Clin. Res. Rev. 2010, 4, 5–9. [Google Scholar] [CrossRef]
- Eshtiaghi, R.; Esteghamati, A.; Nakhjavani, M. Menopause is an independent predictor of metabolic syndrome in Iranian women. Maturitas 2010, 65, 262–266. [Google Scholar] [CrossRef]
- Figueiredo Neto, J.A.; Figuerêdo, E.D.; Barbosa, J.B.; Barbosa Fde, F.; Costa, G.R.; Nina, V.J.; Nina, R.V. Metabolic syndrome and menopause: Cross-sectional study in gynecology clinic. Arq. Bras. Cardiol. 2010, 95, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidari, R.; Sadeghi, M.; Talaei, M.; Rabiei, K.; Mohammadifard, N.; Sarrafzadegan, N. Metabolic syndrome in menopausal transition: Isfahan Healthy Heart Program, a population based study. Diabetol. Metab. Syndr. 2010, 2, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, S.; Srinivas, M.; Agashe, S.; Joshi, J.; Galvankar, P.; Prakasam, C.P.; Vaidya, R. Menopause and metabolic syndrome: A study of 498 urban women from western India. J. Mid-Life Health 2010, 1, 63–69. [Google Scholar] [CrossRef]
- Ruan, X.; Jin, J.; Hua, L.; Liu, Y.; Wang, J.; Liu, S. The prevalence of metabolic syndrome in Chinese postmenopausal women and the optimum body composition indices to predict it. Menopause 2010, 17, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Indhavivadhana, S.; Rattanachaiyanont, M.; Wongvananurak, T.; Kanboon, M.; Techatraisak, K.; Leerasiri, P.; Tanmahasamut, P.; Angsuwathana, S. Predictors for metabolic syndrome in perimenopausal and postmenopausal Thai women. Climacteric J. Int. Menopause Soc. 2011, 14, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Jesmin, S.; Mia, S.; Islam, A.M.; Islam, R.; Sultana, S.N.; Zaedi, S.; Yamaguchi, N.; Okazaki, O.; Moroi, M.; Kimura, S.; et al. Prevalence of metabolic syndrome among rural Bangladeshi women. Diabetes Res. Clin. Pract. 2012, 95, e7–e9. [Google Scholar] [CrossRef]
- Muchanga Sifa, M.J.; Lepira, F.B.; Longo, A.L.; Sumaili, E.K.; Makulo, J.R.; Mbelambela, E.P.; Tozin, R.; Ngatu, N.R.; Suganuma, N. Prevalence and predictors of metabolic syndrome among Congolese pre- and postmenopausal women. Climacteric J. Int. Menopause Soc. 2014, 17, 442–448. [Google Scholar] [CrossRef]
- Marchi, R.; Dell’Agnolo, C.M.; Lopes, T.C.R.; Gravena, A.A.F.; Demitto, M.O.; Brischiliari, S.C.R.; Borghesan, D.H.P.; Carvalho, M.D.B.; Pelloso, S.M. Prevalence of metabolic syndrome in pre- and postmenopausal women. Arch. Endocrinol. Metab. 2017, 61, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Christakis, M.K.; Hasan, H.; De Souza, L.R.; Shirreff, L. The effect of menopause on metabolic syndrome: Cross-sectional results from the Canadian Longitudinal Study on Aging. Menopause 2020, 27, 999–1009. [Google Scholar] [CrossRef]
- Hallajzadeh, J.; Khoramdad, M.; Izadi, N.; Karamzad, N.; Almasi-Hashiani, A.; Ayubi, E.; Qorbani, M.; Pakzad, R.; Hasanzadeh, A.; Sullman, M.J.M.; et al. Metabolic syndrome and its components in premenopausal and postmenopausal women: A comprehensive systematic review and meta-analysis on observational studies. Menopause 2018, 25, 1155–1164. [Google Scholar] [CrossRef]
- Wu, S.I.; Chou, P.; Tsai, S.T. The impact of years since menopause on the development of impaired glucose tolerance. J. Clin. Epidemiol. 2001, 54, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Hwu, C.M.; Lin, Y.C.; Lin, K.H. β-Cell function in postmenopausal women with isolated post-challenge hyperglycemia. J. Diabetes 2018, 10, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ambikairajah, A.; Walsh, E.; Cherbuin, N. Lipid profile differences during menopause: A review with meta-analysis. Menopause 2019, 26, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Dørum, A.; Tonstad, S.; Liavaag, A.H.; Michelsen, T.M.; Hildrum, B.; Dahl, A.A. Bilateral oophorectomy before 50 years of age is significantly associated with the metabolic syndrome and Framingham risk score: A controlled, population-based study (HUNT-2). Gynecol. Oncol. 2008, 109, 377–383. [Google Scholar] [CrossRef]
- Farahmand, M.; Ramezani Tehrani, F.; Bahri Khomami, M.; Noroozzadeh, M.; Azizi, F. Surgical menopause versus natural menopause and cardio-metabolic disturbances: A 12-year population-based cohort study. J. Endocrinol. Investig. 2015, 38, 761–767. [Google Scholar] [CrossRef]
- Ozdemir, S.; Celik, C.; Görkemli, H.; Kiyici, A.; Kaya, B. Compared effects of surgical and natural menopause on climacteric symptoms, osteoporosis, and metabolic syndrome. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2009, 106, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Choi, J.; Park, J.; Lee, J.K.; Shin, A.; Park, S.M.; Kang, D.; Choi, J.Y. Associations of postmenopausal hormone therapy with metabolic syndrome among diabetic and non-diabetic women. Maturitas 2019, 121, 76–82. [Google Scholar] [CrossRef]
- Shakir, Y.A.; Samsioe, G.; Nerbrand, C.; Lidfeldt, J. Combined hormone therapy in postmenopausal women with features of metabolic syndrome. Results from a population-based study of Swedish women: Women’s Health in the Lund Area study. Menopause 2004, 11, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Salpeter, S.R.; Walsh, J.M.; Ormiston, T.M.; Greyber, E.; Buckley, N.S.; Salpeter, E.E. Meta-analysis: Effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes. Metabo. 2006, 8, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Patni, R.; Mahajan, A. The Metabolic Syndrome and Menopause. J. Mid-Life Health. 2018, 9, 111–112. [Google Scholar] [CrossRef]
- Tuysuzoglu, F.N.; Ilhan, G.A.; Yildizhan, B. The impact of surgical menopause on metabolic syndrome, bone mineral density, and vasomotor symptoms. Clin. Exp. Obstet. Gynecol. 2020, 47, 179–182. [Google Scholar] [CrossRef]
- Shufelt, C.L.; Johnson, B.D.; Berga, S.L.; Braunstein, G.D.; Reis, S.E.; Bittner, V.; Yang, Y.; Pepine, C.J.; Sharaf, B.L.; Sopko, G.; et al. Timing of hormone therapy, type of menopause, and coronary disease in women: Data from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Menopause 2011, 18, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Al Kadri, H.; Hassan, S.; Al-Fozan, H.M.; Hajeer, A. Hormone therapy for endometriosis and surgical menopause. Cochrane Database Syst Rev. 2009, 1, CD005997. [Google Scholar] [CrossRef] [PubMed]
- Sarri, G.; Davies, M.; Lumsden, M.A.; Guideline Development Group. Diagnosis and management of menopause: Summary of NICE guidance. BMJ 2015, 351, h5746. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef] [Green Version]
- Stachowiak, G.; Pertyński, T.; Pertyńska-Marczewska, M. Metabolic disorders in menopause. Menopause Rev. 2015, 14, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A. Metabolic syndrome after menopause and the role of hormones. Maturitas 2008, 60, 10–18. [Google Scholar] [CrossRef]
- Bermingham, K.M.; Linenberg, I.; Hall, W.L.; Kadé, K.; Franks, P.W.; Davies, R.; Wolf, J.; Hadjigeorgiou, G.; Asnicar, F.; Segata, N.; et al. Menopause is associated with postprandial metabolism, metabolic health and lifestyle: The ZOE PREDICT study. EBioMedicine 2022, 85, 104303. [Google Scholar] [CrossRef]
- Mankowska, A.; Nowak, L.; Sypniewska, G. Adiponectin and Metabolic Syndrome in Women at Menopause. Ejifcc 2009, 19, 173–184. [Google Scholar] [PubMed]
- Yanes, L.L.; Reckelhoff, J.F. Postmenopausal hypertension. Am. J. Hypertens. 2011, 24, 740–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | Premenopausal | Postmenopausal | p Value |
|---|---|---|---|
| Number of participants | 5976 | 11,484 | - |
| Age, year | 44 ± 6 | 61 ± 6 | <0.001 |
| Waist circumference, cm | 80 ± 10 | 83 ± 10 | <0.001 |
| Body mass index, kg/m2 | 23.6 ± 3.9 | 23.9 ± 3.6 | <0.001 |
| Systolic blood pressure, mmHg | 113 ± 16 | 126 ± 20 | <0.001 |
| Diastolic blood pressure, mmHg | 70 ± 11 | 73 ± 11 | <0.001 |
| Smoke, ever, n (%) | 675 (11) | 609 (5) | <0.001 |
| Alcohol status, ever, n (%) | 224 (4) | 325 (3) | 0.001 |
| Habitual coffee consumption, n (%) | 2830 (47) | 4419 (39) | <0.001 |
| Physical activity, yes, n (%) | 1672 (28) | 6664 (58) | 0.394 |
| Married, yes, n (%) | 5100 (85) | 10,976 (96) | <0.001 |
| Educational status ≥ college, n (%) | 3813 (64) | 3628 (32) | <0.001 |
| Gravidity, yes, n (%) | 5006 (84) | 10,834 (94) | <0.001 |
| Parity, yes, n (%) | 4824 (81) | 10,680 (93) | <0.001 |
| Abortion, yes, n (%) | 2977 (50) | 7150 (62) | <0.001 |
| Breastfeeding, n (%) | 3473 (63) | 7150 (62) | 0.633 |
| Hormone therapy use, n (%) | 472 (8) | 2304 (20) | <0.001 |
| Fasting glucose, mg/dL | 91 ± 17 | 98 ± 21 | <0.001 |
| Triglyceride, mg/dL | 95 ± 103 | 118 ± 74 | <0.001 |
| High-density lipoprotein cholesterol, mg/dL | 58 ± 13 | 58 ± 13 | 0.131 |
| Menopause etiology | |||
| Natural | 9441 (82) | ||
| Surgical | 1967 (17) | ||
| Without information | 76 (1) | ||
| History of chronic kidney disease, n (%) | 13 (0) | 258 (2) | <0.001 |
| Premenopausal (n = 5976) | Postmenopausal (n = 11,484) | p Value | |
|---|---|---|---|
| Presence of metabolic syndrome, n (%) | 841 (14) | 3471 (30) | |
| Age-adjusted OR (95% CI) | 1.0 (reference) | 1.17 (1.03 to 1.33) | 0.019 |
| Multivariable OR (95% CI) | 1.0 (reference) | 1.17 (1.02 to 1.33) | 0.022 |
| Presence of hypertension, n (%) | 1048 (18) | 5425 (47) | |
| Age-adjusted OR (95% CI) | 1.0 (reference) | 0.97 (0.86 to 1.09) | 0.562 |
| Multivariable OR (95% CI) | 1.0 (reference) | 0.96 (0.85 to 1.08) | 0.490 |
| Presence of impaired glucose tolerance, n (%) | 570 (10) | 2991 (26) | |
| Age-adjusted OR (95% CI) | 1.0 (reference) | 1.33 (1.15 to 1.53) | <0.001 |
| Multivariable OR (95% CI) | 1.0 (reference) | 1.30 (1.12 to 1.50) | <0.001 |
| Presence of increased waist circumference, n (%) | 2812 (47) | 6908 (60) | |
| Age-adjusted OR (95% CI) | 1.0 (reference) | 1.03 (0.93 to 1.15) | 0.562 |
| Multivariable OR (95% CI) | 1.0 (reference) | 1.04 (0.94 to 1.16) | 0.438 |
| Presence of hypertriglyceridemia, n (%) | 696 (12) | 2554 (22) | |
| Age-adjusted OR (95% CI) | 1.0 (reference) | 1.83 (1.59 to 2.11) | <0.001 |
| Multivariable OR (95% CI) | 1.0 (reference) | 1.84 (1.60 to 2.12) | <0.001 |
| Presence of low high-density lipoprotein cholesterol, n (%) | 1607 (27) | 3281 (29) | |
| Age-adjusted OR (95% CI) | 1.0 (reference) | 1.03 (0.91 to 1.15) | 0.669 |
| Multivariable OR (95% CI) | 1.0 (reference) | 1.04 (0.92 to 1.17) | 0.558 |
| Type of Menopause | Presence of Metabolic Syndrome, n (%) | Age-Adjusted OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|---|
| Premenopause | 841 (14) | 1.0 (reference) | 1.0 (reference) |
| Natural menopause | 2812 (30) | 1.11 (0.97 to 1.26) | 1.10 (0.96 to 1.23) |
| Surgical menopause | 642 (33) | 1.38 (1.19 to 1.60) | 1.40 (1.20 to 1.63) |
| Postmenopausal Hormone Use | Age-Adjusted OR (95% CI) | p Value | Multivariable OR (95% CI) | p Value |
|---|---|---|---|---|
| Women with natural menopause | ||||
| Non-users | 1.00 (reference) | 1.00 (reference) | ||
| Users | 0.78 (0.69 to 0.087) | <0.001 | 0.79 (0.70 to 0.89) | <0.001 |
| Women with surgical menopause | ||||
| Non-users | 1.00 (reference) | 1.00 (reference) | ||
| Users | 0.83 (0.67 to 1.02) | 0.079 | 0.84 (0.68 to 1.05) | 0.124 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, Y.-J.; Lee, J.-I.; Huang, S.-P.; Chen, S.-C.; Geng, J.-H.; Su, C.-H. Association between Menopause, Postmenopausal Hormone Therapy and Metabolic Syndrome. J. Clin. Med. 2023, 12, 4435. https://doi.org/10.3390/jcm12134435
Ou Y-J, Lee J-I, Huang S-P, Chen S-C, Geng J-H, Su C-H. Association between Menopause, Postmenopausal Hormone Therapy and Metabolic Syndrome. Journal of Clinical Medicine. 2023; 12(13):4435. https://doi.org/10.3390/jcm12134435
Chicago/Turabian StyleOu, Ying-Ju, Jia-In Lee, Shu-Pin Huang, Szu-Chia Chen, Jiun-Hung Geng, and Chia-Hung Su. 2023. "Association between Menopause, Postmenopausal Hormone Therapy and Metabolic Syndrome" Journal of Clinical Medicine 12, no. 13: 4435. https://doi.org/10.3390/jcm12134435
APA StyleOu, Y. -J., Lee, J. -I., Huang, S. -P., Chen, S. -C., Geng, J. -H., & Su, C. -H. (2023). Association between Menopause, Postmenopausal Hormone Therapy and Metabolic Syndrome. Journal of Clinical Medicine, 12(13), 4435. https://doi.org/10.3390/jcm12134435