Catheter-Based Techniques for Addressing Atrioventricular Valve Regurgitation in Adult Congenital Heart Disease Patients: A Descriptive Cohort
Abstract
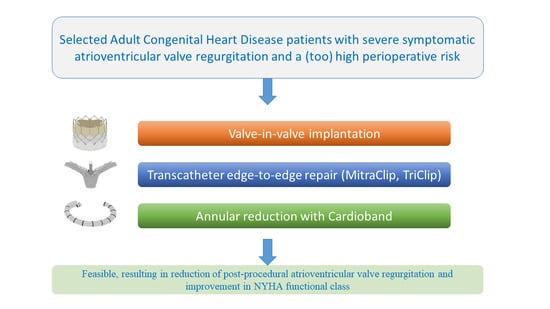
:1. Introduction
2. Methods
Statistical Analysis
3. Results
3.1. Patient A
3.2. Patient B
3.3. Patient C
3.4. Patient D
3.5. Patient E
4. Discussion
4.1. Feasible and Patient Tailored Alternative to Surgery
4.2. Dedicated ACHD Heart Team
4.3. Peri-Procedural Imaging and Potential for Virtual Reality
4.4. Study Limitations
5. Conclusions and Clinical Implication
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclosures
References
- Bouma, B.J.; Mulder, B.J. Changing Landscape of Congenital Heart Disease. Circ. Res. 2017, 120, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Erikssen, G.; Liestøl, K.; Seem, E.; Birkeland, S.; Saatvedt, K.J.; Hoel, T.N.; Døhlen, G.; Skulstad, H.; Svennevig, J.L.; Thaulow, E.; et al. Achievements in congenital heart defect surgery: A prospective, 40-year study of 7038 patients. Circulation 2015, 131, 337–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutluer, F.O.; Çeliker, A. General Concepts in Adult Congenital Heart Disease. Balk. Med. J. 2018, 35, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Stefanescu Schmidt, A.C.; Horlick, E.; Aboulhosn, J. Transcatheter Interventions in Patients with Adult Congenital Heart Disease. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100438. [Google Scholar] [CrossRef]
- Barry, O.M.; Bouhout, I.; Kodali, S.K.; George, I.; Rosenbaum, M.S.; Petit, C.J.; Kalfa, D. Interventions for Congenital Atrioventricular Valve Dysfunction: JACC Focus Seminar. J. Am. Coll. Cardiol. 2022, 79, 2259–2269. [Google Scholar] [CrossRef] [PubMed]
- Schamroth Pravda, N.; Vaknin Assa, H.; Sondergaard, L.; Bajoras, V.; Sievert, H.; Piayda, K.; Levi, A.; Witberg, G.; Shapira, Y.; Hamdan, A.; et al. Transcatheter Interventions for Atrioventricular Dysfunction in Patients with Adult Congenital Heart Disease: An International Case Series. J. Clin. Med. 2023, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Silini, A.; Iriart, X. Percutaneous edge-to-edge repair in congenital heart disease: Preliminary results of a promising new technique. Int. J. Cardiol. Congenit. Heart Dis. 2022, 8, 100370. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.; Berzingi, C.O.; Aljohani, S.; Hijazi, M.; Al-Hallak, A.; Alkhouli, M. Contemporary Trends in the Use and Outcomes of Surgical Treatment of Tricuspid Regurgitation. J. Am. Heart Assoc. 2017, 6, e007597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickenig, G.; Weber, M.; Schüler, R.; Hausleiter, J.; Nabauer, M.; von Bardeleben, R.S.; Sotiriou, E.; Schäfer, U.; Deuschl, F.; Alessandrini, H.; et al. Tricuspid valve repair with the Cardioband system: Two-year outcomes of the multicentre, prospective TRI-REPAIR study. EuroIntervention 2021, 16, e1264–e1271. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.A.; Abramson, S.V.; Lim, S.; Fowler, D.; Smith, R.L.; Grayburn, P.A.; Kodali, S.K.; Hahn, R.T.; Kipperman, R.M.; Koulogiannis, K.P.; et al. 1-Year Outcomes of Cardioband Tricuspid Valve Reconstruction System Early Feasibility Study. JACC Cardiovasc. Interv. 2022, 15, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Taggart, N.W.; Cabalka, A.K.; Eicken, A.; Aboulhosn, J.A.; Thomson, J.D.R.; Whisenant, B.; Bocks, M.L.; Schubert, S.; Jones, T.K.; Asnes, J.D.; et al. Outcomes of Transcatheter Tricuspid Valve-In-Valve Implantation in Patients with Ebstein Anomaly. Am. J. Cardiol. 2018, 121, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Kodali, S.; Fam, N.; Bapat, V.; Bartus, K.; Rodés-Cabau, J.; Dagenais, F.; Estevez-Loureiro, R.; Forteza, A.; Kapadia, S.; et al. Early Multinational Experience of Transcatheter Tricuspid Valve Replacement for Treating Severe Tricuspid Regurgitation. JACC Cardiovasc. Interv. 2020, 13, 2482–2493. [Google Scholar] [CrossRef] [PubMed]
- Ott, I.; Rumpf, P.M.; Kasel, M.; Kastrati, A.; Kaemmerer, H.; Schunkert, H.; Ewert, P.; Tutarel, O. Transcatheter valve repair in congenitally corrected transposition of the great arteries. EuroIntervention 2021, 17, 744–746. [Google Scholar] [CrossRef] [PubMed]
- Blusztein, D.; Moore, P.; Qasim, A.; Mantri, N.; Mahadevan, V.S. Transcatheter Edge-To-Edge Repair of Systemic Tricuspid Valve in Extracardiac Fontan Circulation. J. Am. Coll. Cardiol. Case Rep. 2022, 4, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Guerin, P.; Jalal, Z.; Le Ruz, R.; Cueff, C.; Hascoet, S.; Bouvaist, H.; Ladouceur, M.; Levy, F.; Hugues, N.; Malekzadeh-Milani, S.G.; et al. Percutaneous Edge-To-Edge Repair for Systemic Atrioventricular Valve Regurgitation in Patients with Congenital Heart Disease: The First Descriptive Cohort. J. Am. Heart Assoc. 2022, 1, e025628. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.M.; Jolley, M.A.; Mascio, C.E.; Chen, J.M.; Fuller, S.; Rome, J.J.; Silvestro, E.; Whitehead, K.K. Clinical 3D modeling to guide pediatric cardiothoracic surgery and intervention using 3D printed anatomic models, computer aided design and virtual reality. 3D Print. Med. 2022, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Krishnan, A.; Huang, C.Y.; Spevak, P.; Vricella, L.; Hibino, N.; Garcia, J.R.; Gaur, L. Role of virtual reality in congenital heart disease. Congenit. Heart Dis. 2018, 13, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.A.; Vardanyan, R.; Lopuszko, A.; Alt, C.; Stoffels, I.; Schmack, B.; Ruhparwar, A.; Zhigalov, K.; Zubarevich, A.; Weymann, A. Virtual and Augmented Reality in Cardiac Surgery. Braz. J. Cardiovasc. Surg. 2022, 37, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Mahtab, E.A.F.; Egorova, A.D. Current and future applications of virtual reality technology for cardiac interventions. Nat. Rev. Cardiol. 2022, 19, 779–780. [Google Scholar] [CrossRef] [PubMed]







| Patient A | Patient B | Patient C | Patient D | Patient E | |
|---|---|---|---|---|---|
| Age (years) | 75 | 40 | 48 | 52 | 72 |
| Sex | male | female | male | male | female |
| CHD diagnosis and surgical history | BAV with severe aortic regurgitation, Ross at the age of 25 and ascending aorta replacement and AVR bioprosthesis due to autograft failure at the age of 65 | M. Ebstein with severe tricuspid regurgitation right-left shunt over an open PFO, TV annuloplasty followed by replacement with a bioprosthesis at the age of 31 | Left isomerism, DORV-Fallot type, hypoplastic left ventricle, mitral valve atresia, right modified Blalock–Taussig shunt at the age of 1, left modified Blalock–Tausig shunt at the age of 13, aorto-pulmonary shunt to the right pulmonary artery at the age of 20 because of occlusion of the left Blalock–Taussig shunt | Left isomerism, DORV-TGA, large ASD, PS, right-sided aortic arch, persistent left SVC, bilateral bi-directional Glenn anastomosis at the age of 31 | Tetralogy of Fallot, Blalock shunt at the age of 4 and surgical correction at the age of 22, pulmonary homograft implantation at the age of 58, numerous ablation procedures for atrial fibrillation and flutters, chronic RV pacing due to a high degree AV-block |
| Concomitant cardiac lesions and diagnoses | Paroxysmal atrial fibrillation | None | Multiple systemic to pulmonary artery shunts, abnormal venous return with hemiazygos continuation of the IVC and a persistent left SVC, paroxysmal atrial fibrillation | Persistence of left SVC, abnormal venous return with azygos continuation of the IVC, permanent accepted atrial fibrillation, endocarditis | Permanent accepted atrial fibrillation |
| Number of previous cardiac surgeries | 2 | 2 | 3 | 1 | 3 |
| Non-cardiac comorbidities | Bilateral pulmonary embolism, hypertenstion | - | - | Epilepsy | Epilepsy |
| Morphology of the systemic ventricle | left | left | right | right | left |
| Pre-procedural NYHA class | III | II | IV | III | IV |
| Morphology of the regurgitant AV valve | TV (subpulmonary) | TV (subpulmonary) | TV (systemic) | common AV-valve (systemic) | TV (subpulmonary) |
| Severity of AV valve regurgitation | IV+/torrential | IV/severe | IV+/torrential | IV/severe | IV/severe |
| (Dominant) mechanism of AV valve regurgitation | Annulus dilatation | Bioprosthesis degeneration | Annulus dilatation | Annulus dilatation | Annulus dilatation, impingement by device lead |
| Main imaging findings | Moderately reduced left and right ventricular function, dilated RV, severe/torrential TR and right atrial dilatation, estimated filling pressures 20 mmHg | Moderately reduced right ventricular function | Dilated ventricle with impaired systolic function, severely dilated functional mono-atrium | Severely dilated functional mono-atrium, extensive network of coronary fistulae, Moderalety reduced systolic and diastolic systemic ventricular function | Preserved right ventricular function |
| Renal function (eGFR) | 63 mL/min/1.73 m2 | 85 mL/min/1.73 m2 | 38 mL/min/1.73 m2 | >90 mL/min/1.73 m2 | 89 mL/min/1.73 m2 |
| Cardiac pharmacotherapy | Vitamin K antagonist, sotalol, ACE-inhibitor, aldosterone receptor antagonist, loop diuretic, calcium antagonist, statin | None | Amiodarone, DOAC, aldosterone receptor antagonist, loop diuretic | Aldosterone receptor antagonist, DOAC, loop diuretic | Vitamin K antagonist, sotalol, aldosterone receptor antagonist, loop diuretic |
| Vascular access | Right femoral venous access | Right femoral venous access | Right transjugular venous access | Right lateral thoracotomy through the 5th intercostal space | Right femoral venous access |
| Intervention | Annuloplasty (Cardioband) | Valve-in-valve implantation (Sapien 3) | TEER (Triclip), two XTW clips between A-S leaflets | Hybrid TEER (MitraClip), two XTW clips between A-P leaflets | Annuloplasty (Cardioband) TEER (Triclip), two XTW clips |
| Procedural complications | None | None | None | None | None |
| Post-procedural AVVR grade | II | <I | II | II | I-II |
| Post-procedural NYHA class | I-II | I | III | II | II |
| NYHA class at latest follow-up | I-II | I | III | IV | II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Bouziani, A.; Witte, L.S.; Bouma, B.J.; Jongbloed, M.R.M.; Robbers-Visser, D.; Straver, B.; Beijk, M.A.M.; Kiès, P.; Koolbergen, D.R.; van der Kley, F.; et al. Catheter-Based Techniques for Addressing Atrioventricular Valve Regurgitation in Adult Congenital Heart Disease Patients: A Descriptive Cohort. J. Clin. Med. 2023, 12, 4798. https://doi.org/10.3390/jcm12144798
El Bouziani A, Witte LS, Bouma BJ, Jongbloed MRM, Robbers-Visser D, Straver B, Beijk MAM, Kiès P, Koolbergen DR, van der Kley F, et al. Catheter-Based Techniques for Addressing Atrioventricular Valve Regurgitation in Adult Congenital Heart Disease Patients: A Descriptive Cohort. Journal of Clinical Medicine. 2023; 12(14):4798. https://doi.org/10.3390/jcm12144798
Chicago/Turabian StyleEl Bouziani, Abdelhak, Lars S. Witte, Berto J. Bouma, Monique R. M. Jongbloed, Daniëlle Robbers-Visser, Bart Straver, Marcel A. M. Beijk, Philippine Kiès, David R. Koolbergen, Frank van der Kley, and et al. 2023. "Catheter-Based Techniques for Addressing Atrioventricular Valve Regurgitation in Adult Congenital Heart Disease Patients: A Descriptive Cohort" Journal of Clinical Medicine 12, no. 14: 4798. https://doi.org/10.3390/jcm12144798
APA StyleEl Bouziani, A., Witte, L. S., Bouma, B. J., Jongbloed, M. R. M., Robbers-Visser, D., Straver, B., Beijk, M. A. M., Kiès, P., Koolbergen, D. R., van der Kley, F., Schalij, M. J., de Winter, R. J., & Egorova, A. D. (2023). Catheter-Based Techniques for Addressing Atrioventricular Valve Regurgitation in Adult Congenital Heart Disease Patients: A Descriptive Cohort. Journal of Clinical Medicine, 12(14), 4798. https://doi.org/10.3390/jcm12144798