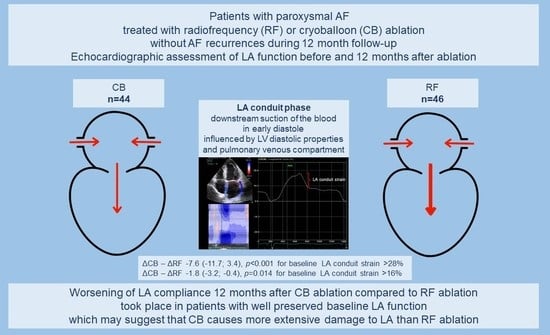
Subclinical Dysfunction of Left Atrial Compliance after Cryoballoon versus Radiofrequency Ablation for Paroxysmal Atrial Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiography
2.3. Catheter Ablation Procedure
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
Patient Characteristics
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association of cardio-thoracic surgery (EACTS): The Task Force for the diagnosis and man-agement of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Donal, E.; Behagel, A.; Feneon, D. Value of left atrial strain: A highly promising field of investigation. Eur. Heart J.-Cardiovasc. Imaging 2014, 16, 356–357. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Yui, Y.; Kimata, A.; Koda, N.; Kato, J.; Baba, M.; Misaki, M.; Abe, D.; Tokunaga, C.; Akishima, S.; et al. Troponin elevation after radiofrequency catheter ablation of atrial fibrillation: Relevance to AF substrate, procedural outcomes, and reverse structural remodeling. Heart Rhythm 2014, 11, 1336–1342. [Google Scholar] [CrossRef]
- Packer, M. Effect of catheter ablation on pre-existing abnormalities of left atrial systolic, diastolic, and neurohormonal functions in patients with chronic heart failure and atrial fibrillation. Eur. Heart J. 2019, 40, 1873–1879. [Google Scholar] [CrossRef]
- Kuck, K.-H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.J.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef]
- Antolič, B.; Pernat, A.; Cvijić, M.; Žižek, D.; Jan, M.; Šinkovec, M. Radiofrequency catheter ablation versus balloon cryoablation of atrial fibrillation: Markers of myocardial damage, inflammation, and thrombogenesis. Wien. Klin. Wochenschr. 2016, 128, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Siklódy, C.H.; Arentz, T.; Minners, J.; Jesel, L.; Stratz, C.; Valina, C.M.; Weber, R.; Kalusche, D.; Toti, F.; Morel, O.; et al. Cellular damage, platelet activation, and inflammatory response after pulmonary vein isolation: A randomized study comparing radiofrequency ablation with cryoablation. Heart Rhythm 2012, 9, 189–196. [Google Scholar] [CrossRef]
- Yano, M.; Egami, Y.; Yanagawa, K.; Nakamura, H.; Matsuhiro, Y.; Yasumoto, K.; Tsuda, M.; Okamoto, N.; Tanaka, A.; Matsunaga-Lee, Y.; et al. Comparison of myocardial injury and inflammation after pulmonary vein isolation for paroxysmal atrial fibrillation between radiofrequency catheter ablation and cryoballoon ablation. J. Cardiovasc. Electrophysiol. 2020, 31, 1315–1322. [Google Scholar] [CrossRef]
- Pilichowska-Paszkiet, E.M.; Baran, J.; Kułakowski, P.; Zaborska, B. Echocardiographic assessment of left atrial function for prediction of efficacy of catheter ablation for atrial fibrillation. Medicine 2021, 100, e27278. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, A.; Pilichowska-Paszkiet, E.; Zuk, A.; Piotrowski, R.; Kryński, T.; Baran, J.; Zaborska, B.; Kułakowski, P. Acceleration of sinus rhythm following ablation for atrial fibrillation: A simple parameter predicting ablation efficacy. Kardiol. Pol. 2019, 77, 960–965. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to stand-ardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, Y.E.; Kim, S.-A.; Kim, S.H.; Park, J.-H.; Park, K.-H.; Choi, S.; Kim, M.-K.; Kim, H.-S.; Cho, G.-Y. Left atrial mechanical function and stiffness in patients with paroxysmal atrial fibrillation. J. Cardiovasc. Ultrasound 2012, 20, 140–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.-H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. EP Eur. 2018, 20, 157–208. [Google Scholar] [CrossRef]
- Vieira, M.J.; Teixeira, R.; Gonçalves, L.; Gersh, B.J. Left atrial mechanics: Echocardiographic assessment and clinical implications. J. Am. Soc. Echocardiogr. 2014, 27, 463–478. [Google Scholar] [CrossRef]
- Marino, P.N. Left atrial conduit function: A short review. Physiol. Rep. 2021, 9, e15053. [Google Scholar] [CrossRef]
- Marino, P.N.; Zanaboni, J.; Degiovanni, A.; Sartori, C.; Patti, G.; Fraser, A.G. Left atrial conduit flow rate at baseline and during exercise: An index of impaired relaxation in HFpEF patients. ESC Heart Fail. 2021, 8, 4334–4342. [Google Scholar] [CrossRef]
- Andrade, J.G.; Wazni, O.M.; Kuniss, M.; Hawkins, N.M.; Deyell, M.W.; Chierchia, G.B.; Nissen, S.; Verma, A.; Wells, G.A.; Turgeon, R.D. Cryoballoon Ablation as Initial Treatment for Atrial Fibrillation: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 914–930. [Google Scholar] [CrossRef]
- You, L.; Yao, L.; Zhou, B.; Jin, L.; Yin, H.; Wu, J.; Yin, G.; Yang, Y.; Zhang, C.; Liu, Y.; et al. Effects of different ablation strategies on long-term left atrial function in patients with paroxysmal atrial fibrillation: A single-blind randomized controlled trial. Sci. Rep. 2019, 9, 7695. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Romero, D.; Marín, F.; Roldán, V.; Peñafiel, P.; Vilchez, J.A.; Orenes-Piñero, E.; Giner, J.A.; Valdés, M.; García-Alberola, A. Comparative determination and monitoring of biomarkers of necrosis and myocardial remodeling between radiofrequency ablation and cryoablation. Pacing Clin. Electrophysiol. 2012, 36, 31–36. [Google Scholar] [CrossRef]
- Kühne, M.; Suter, Y.; Altmann, D.; Ammann, P.; Schaer, B.; Osswald, S.; Sticherling, C. Cryoballoon versus radiofrequency catheter ablation of paroxysmal atrial fibrillation: Bi-omarkers of myocardial injury, recurrence rates, and pulmonary vein reconnection patterns. Heart Rhythm 2010, 7, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Dhorepatil, A.; Kreidieh, O.; Mekhael, M.; Noujaim, C.; Assaf, A.; Feng, H.; Marrouche, N. Differences in postablation cardiac MRI scar between radiofrequency and cry-oballoon ablation: A DECAAF II subanalysis. J. Cardiovasc. Electrophysiol. 2023, 34, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, B.; Qiu, C.; Han, Z.; Wang, X.; Lu, W.; Chen, X.; Chen, Y.; Pan, L.; Sun, G.; et al. The effect of left atrial remodeling after cryoballoon ablation and radiofrequency ablation for paroxysmal atrial fibrillation. Clin. Cardiol. 2021, 44, 78–84. [Google Scholar] [CrossRef]
- Giannopoulos, G.; Kossyvakis, C.; Vrachatis, D.; Aggeli, C.; Tsitsinakis, G.; Letsas, K.; Tsiachris, D.; Tsoukala, S.; Efremidis, M.; Katritsis, D.; et al. Effect of cryoballoon and radiofrequency ablation for pulmonary vein isolation on left atrial function in patients with nonvalvular paroxysmal atrial fibrillation: A prospective randomized study (Cryo-LAEF study). J. Cardiovasc. Electrophysiol. 2019, 30, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Kuppahally, S.S.; Akoum, N.; Badger, T.J.; Burgon, N.S.; Haslam, T.; Kholmovski, E.; Macleod, R.; McGann, C.; Marrouche, N.F. Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am. Heart J. 2010, 160, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Gerach, T.; Schuler, S.; Wachter, A.; Loewe, A. The Impact of Standard Ablation Strategies for Atrial Fibrillation on Cardiovascular Performance in a Four-Chamber Heart Model. Cardiovasc. Eng. Technol. 2023, 14, 296–314. [Google Scholar] [CrossRef]
- Sielski, J.; Grabowska, U.; Kaziród-Wolski, K.; Matejko, M. Correlation analysis of the relationship between B-type natriuretic peptide and selected echocardiographic parameters in patients with permanent pacemakers. Med. Stud. 2015, 4, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Luchner, A.; Möckel, M.; Spanuth, E.; Möcks, J.; Peetz, D.; Baum, H.; Spes, C.; Wrede, C.E.; Vollert, J.; Müller, R.; et al. N-terminal pro brain natriuretic peptide in the management of patients in the medical emergency department (PROMPT): Correlation with disease severity, utilization of hospital resources, and prognosis in a large, prospective, randomized multicentre trial. Eur. J. Heart Fail. 2012, 14, 259–267. [Google Scholar] [CrossRef]
- Sardana, M.; Syed, A.A.; Hashmath, Z.; Phan, T.S.; Koppula, M.R.; Kewan, U.; Ahmed, Z.; Chandamuri, R.; Varakantam, S.; Shah, E.; et al. Beta-Blocker Use Is Associated With Impaired Left Atrial Function in Hypertension. J. Am. Heart Assoc. 2017, 6, e005163. [Google Scholar] [CrossRef] [Green Version]

| Clinical Characteristic | Study Group N = 90 | RF N = 46 (51%) | CB N = 44 (49%) | RF vs. CB p |
|---|---|---|---|---|
| Men n (%) | 59 (65.6%) | 34 (73.9%) | 25 (56.8%) | 0.09 |
| Age (years) | 57.2 ± 9.7 | 56.2 ± 10.1 | 59.2 ± 9.3 | 0.35 |
| BMI (kg/m2) | 29.0 ± 3.5 | 29.2 ± 3.0 | 28.8 ± 4.0 | 0.63 |
| Duration of AF (years) | 3.0 (1.6–8.0) | 3.0 (2.0–7.0) | 2.0 (1.5–10) | 0.39 |
| DM | 4 (9.1%) | 3 (14.3%) | 1 (4.3%) | 0.34 |
| CAD | 6 (6.7%) | 3 (6.5%) | 3 (6.8%) | 1.00 |
| Arterial hypertension | 59 (65.6%) | 28 (60.9%) | 31 (70.4%) | 0.34 |
| Scale CHA2DS2-VASc | 1 (1–2) | 1 (0–2) | 2 (1–2) | 0.09 |
| Hyperlipidemia | 36 (40%) | 20 (43.5%) | 16 (36.4%) | 0.49 |
| OSA | 4 (4.4%) | 2 (4.3%) | 2 (4.5%) | 1.00 |
| Systolic BP (mmHg) | 133.1 ± 11.2 | 133.0 ± 12.4 | 133.3 ± 9.9 | 0.90 |
| Diastolic BP (mmHg) | 86.4 ± 7.0 | 85.9 ± 7.2 | 86.9 ± 6.9 | 0.47 |
| B-blockers | 55 (61.8%) | 36 (78.3%) | 19 (44.2%) | 0.0009 |
| AA | 53 (76.8%) | 32 (84.2%) | 21 (67.7%) | 0.11 |
| ACE-I | 46 (51.1%) | 19 (41.3%) | 27 (61.4%) | 0.06 |
| Parameter | Baseline | 12 Months | Δ 95% CI | p |
|---|---|---|---|---|
| LVEDd (mm) | 48.5 ± 4.5 | 47.3 ± 4.6 | −1.2 (−1.8; −0.5) | <0.001 |
| LVEF (%) | 63.1 ± 6.9 | 62.5 ± 3.9 | −0.6 (−1.6; 0.4) | 0.23 |
| LAd (mm) | 38.3 ± 4.1 | 36.8 ± 3.6 | −1.5 (−2.2; −0.9) | <0.001 |
| LAV index (mL/m2) | 34.0 ± 12.2 | 32.7 ± 8.4 | −1.3 (−3.1; 0.6) | 0.17 |
| Mitral E (cm/s) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.0 (0.0; 0.1) | 0.03 |
| Mitral A (cm/s) | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.0 (−0.0; 0.5) | 0.31 |
| e′ (cm/s) | 9.0 ± 1.9 | 9.4 ± 2.1 | 0.5 (0.2; 0.8) | 0.004 |
| a′ (cm/s) | 8.6 ± 2.1 | 9.0 ± 1.8 | 0.4 (0.0; 0.7) | 0.04 |
| E/e′ | 7.2 ± 2.3 | 7.7 ± 1.9 | 0.0 (−0.4; 0.3) | 0.81 |
| LASr (%) | 27.8 ± 6.9 | 27.8 ± 6.2 | 0.0 (−1.3; 1.1) | 0.91 |
| LAScd (%) | 15.0 ± 4.8 | 14.2 ± 4.2 | −0.8 (−1.7; 0.1) | 0.06 |
| LASct (%) | 12.8 ± 4.5 | 13.6 ± 3.7 | 0.8 (−0.3; 1.8) | 0.14 |
| LASRr (s−1) | 1.1 ± 0.3 | 1.3 ± 0.3 | 0.1 (0.1; 0.2) | <0.001 |
| LASRcd (s−1) | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.1 (0.1; 0.2) | <0.001 |
| LASRct (s−1) | 1.4 ± 0.5 | 1.5 ± 0.7 | 0.0 (−0.0; 0.2) | 0.20 |
| LA stiffness | 0.3 ± 0.2 | 0.3 ± 0.1 | −0.0 (−0.1; 0.0) | 0.27 |
| Parameter | RF | CB | CB vs. RF | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Months | Adj. Δ (95% CI) 12m—Baseline | Baseline | 12 Months | Adj. Δ (95% CI) 12m—Baseline | Adj. Δ CB–Adj. Δ RF | p | |
| LAd (mm) | 38.4 ± 4.0 | 36.9 ± 3.8 | −1.5 (−2.3; −0.7) * | 38.1 ± 4.2 | 36.6 ± 3.3 | −1.6 (−2.4; −0.7) * | 0.0 (−1.2; 1.1) | 0.94 |
| LAV index (mL/m2) | 34.3 ± 9.9 | 33.1 ± 8.8 | −1.1 (−2.8; 0.7) | 33.6 ± 14.6 | 32.3 ± 8.0 | −1.5 (−3.4; 0.4) | −0.4 (−3.0; 2.2) | 0.75 |
| Mitral E (cm/s) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.0 (0.0; 0.1) | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.0 (0.0; 0.1) | 0.00 (−0.1; 0.1) | 0.99 |
| Mitral A (cm/s) | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.0 (0.0; 0.0) | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.0 (0.0; 0.1) | 0.1 (0.0; 0.10) | 0.07 |
| e′ (cm/s) | 8.9 ± 2.1 | 9.5 ± 2.5 | 0.6 (0.2; 1.1) | 9.1 ± 1.7 | 9.3 ± 1.7 | 0.3 (−0.2; 0.7) | −0.4 (−1.0; 0.2) | 0.23 |
| a′ (cm/s) | 8.8 ± 1.8 | 9.1 ± 1.6 | 0.3 (−0.1; 0.7) | 8.4 ± 2.3 | 8.9 ± 2.0 | 0.5 (0.0; 0.5) * | 0.1 (−0.5; 0.7) | 0.67 |
| E/e′ | 7.8 ± 2.4 | 7.6 ± 2.1 | −0.1 (−0.5; 0.3) | 7.7 ± 2.2 | 7.7 ± 1.8 | 0.0 (−0.4; 0.5) | 0.2 (−0.5; 0.8) | 0.62 |
| LASr (%) | 27.8 ± 6.7 | 28.0 ± 6.1 | 0.2 (−1.3; 1.6) | 27.8 ± 7.4 | 27.5 ± 6.4 | −0.3 (−1.9; 1.2) | −0.5 (−2.6; 1.6) | 0.65 |
| LAScd (%) | 14.7 ± 4.1 | 14.6 ± 4.8 | −0.2 (−1.2; 0.8) | 15.3 ± 5.4 | 13.7 ± 3.4 | 0.26 (−0.18; 0.70) | −1.3 (−2.8; 0.2) | 0.09 |
| LASct (%) | 13.1 ± 4.9 | 13.4 ± 3.4 | 0.5 (−0.6; 1.5) | 12.5 ± 3.9 | 13.8 ± 4.1 | 1.1 (−0.0; 2.3) | 0.7 (−0.9; 2.3) | 0.40 |
| LASRr (s−1) | 1.2 ± 0.22 | 1.3 ± 0.31 | 0.1 (0.0; 0.2) * | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.1 (0.1; 0.2) * | 0.0 (0.1; 0.2) | 0.52 |
| LASRcd (s−1) | 1.1 ± 0.3 | 1.3 ± 0.4 | 0.2 (0.1; 0.2) * | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.1 (0.0; 0.2) * | 0.0 (−0.2; 0.1) | 0.63 |
| LASRct (s−1) | 1.5 ± 0.4 | 1.5 ± 0.3 | 0.1 (0.0; 0.2) | 1.4 ± 0.5 | 1.5 ± 0.4 | 0.0 (−0.1; 0.2) | 0.0 (−0.2; 0.1) | 0.60 |
| LA stiffness | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.0 (−0.1; 0.0) | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.0 (−0.1; 0.0) | 0.0 (0.0; 0.1) | 0.68 |
| Parameter | RF | CB | CB vs. RF | |||
|---|---|---|---|---|---|---|
| Baseline Tested Values | Adj. Δ [95% CI] 12m—Baseline | Baseline Tested Values | Adj. Δ [95% CI] 12m—Baseline | Adj. Δ CB—Adj. Δ RF | p | |
| e′ (cm/s) | 5 8 12 | 0.7 (−0.2; 1.5) 0.7 (0.2; 1.1) * 0.6 (−0.1; 1.3) | 5 8 12 | 2.1 (1.0; 3.2) * 0.7 (0.2; 1.2) * −1.1 (−1.9; −0.2) * | 1.4 (0.0; 2.8) 0.1 (−0.6; 0.7) −1.7 (−2.8; −0.6) * | 0.05 0.83 0.003 |
| LAScd (%) | 8 16 28 | 0.8 (−1.0; 2.6) −0.3 (−1.3; 0.7) −1.9 (−5.1; 1.4) | 8 16 28 | 2.9 (1.1; 4.6) * −2.1 (−3.1; −1.1) * −9.5 (−12.0; −6.9) * | 2.1 (−0.5; 4.5) −1.8 (−3.2; −0.4) * −7.6 (−11.7; −3.4) * | 0.11 0.014 <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilichowska-Paszkiet, E.; Sikorska, A.; Kowalik, I.; Smarż, K.; Sikora-Frąc, M.; Baran, J.; Piotrowski, R.; Kryński, T.; Kułakowski, P.; Zaborska, B. Subclinical Dysfunction of Left Atrial Compliance after Cryoballoon versus Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. J. Clin. Med. 2023, 12, 4974. https://doi.org/10.3390/jcm12154974
Pilichowska-Paszkiet E, Sikorska A, Kowalik I, Smarż K, Sikora-Frąc M, Baran J, Piotrowski R, Kryński T, Kułakowski P, Zaborska B. Subclinical Dysfunction of Left Atrial Compliance after Cryoballoon versus Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. Journal of Clinical Medicine. 2023; 12(15):4974. https://doi.org/10.3390/jcm12154974
Chicago/Turabian StylePilichowska-Paszkiet, Ewa, Agnieszka Sikorska, Ilona Kowalik, Krzysztof Smarż, Małgorzata Sikora-Frąc, Jakub Baran, Roman Piotrowski, Tomasz Kryński, Piotr Kułakowski, and Beata Zaborska. 2023. "Subclinical Dysfunction of Left Atrial Compliance after Cryoballoon versus Radiofrequency Ablation for Paroxysmal Atrial Fibrillation" Journal of Clinical Medicine 12, no. 15: 4974. https://doi.org/10.3390/jcm12154974
APA StylePilichowska-Paszkiet, E., Sikorska, A., Kowalik, I., Smarż, K., Sikora-Frąc, M., Baran, J., Piotrowski, R., Kryński, T., Kułakowski, P., & Zaborska, B. (2023). Subclinical Dysfunction of Left Atrial Compliance after Cryoballoon versus Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. Journal of Clinical Medicine, 12(15), 4974. https://doi.org/10.3390/jcm12154974