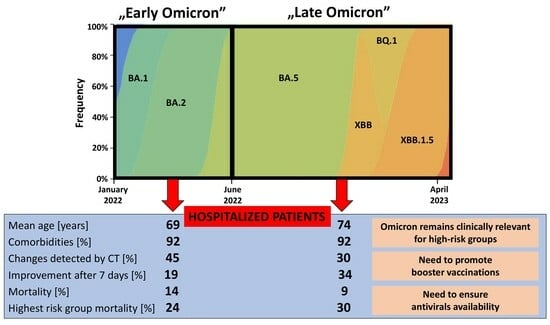
Change in the Clinical Picture of Hospitalized Patients with COVID-19 between the Early and Late Period of Dominance of the Omicron SARS-CoV-2 Variant
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tracking SARS-CoV-2 Variants. 2023. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants/tracking-SARS-CoV-2-variants (accessed on 16 June 2023).
- WHO Coronavirus (COVID-19) Dashboard. 2023. Available online: https://covid19.who.int/ (accessed on 16 June 2023).
- Tian, D.; Sun, Y.; Xu, H.; Ye, Q. The Emergence and Epidemic Characteristics of the Highly Mutated SARS-CoV-2 Omicron Variant. J. Med. Virol. 2022, 94, 2376–2383. [Google Scholar] [CrossRef]
- Hu, F.-H.; Jia, Y.-J.; Zhao, D.-Y.; Fu, X.-L.; Zhang, W.-Q.; Tang, W.; Hu, S.-Q.; Wu, H.; Ge, M.-W.; Du, W.; et al. Clinical Outcomes of the Severe Acute Respiratory Syndrome Coronavirus 2 Omicron and Delta Variant: Systematic Review and Meta-Analysis of 33 Studies Covering 6 037 144 Coronavirus Disease 2019-Positive Patients. Clin. Microbiol. Infect. 2023, 29, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Bhattacharya, M.; Nag, S.; Dhama, K.; Chakraborty, C. A Detailed Overview of SARS-CoV-2 Omicron: Its Sub-Variants, Mutations and Pathophysiology, Clinical Characteristics, Immunological Landscape, Immune Escape, and Therapies. Viruses 2023, 15, 167. [Google Scholar] [CrossRef]
- Bouzid, D.; Visseaux, B.; Kassasseya, C.; Daoud, A.; Fémy, F.; Hermand, C.; Truchot, J.; Beaune, S.; Javaud, N.; Peyrony, O.; et al. Comparison of Patients Infected with Delta Versus Omicron COVID-19 Variants Presenting to Paris Emergency Departments: A Retrospective Cohort Study. Ann. Intern. Med. 2022, 175, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical Severity of, and Effectiveness of MRNA Vaccines against, Covid-19 from Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: Prospective Observational Study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef]
- Ao, D.; He, X.; Hong, W.; Wei, X. The Rapid Rise of SARS-CoV-2 Omicron Subvariants with Immune Evasion Properties: XBB.1.5 and BQ.1.1 Subvariants. MedComm 2023, 4, e239. [Google Scholar] [CrossRef]
- Hyams, C.; Challen, R.; Marlow, R.; Nguyen, J.; Begier, E.; Southern, J.; King, J.; Morley, A.; Kinney, J.; Clout, M.; et al. Severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 Infection among Hospitalised Adults: A Prospective Cohort Study in Bristol, United Kingdom. Lancet Reg. Health Eur. 2023, 25, 100556. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhi, H.; Teng, Y. The Outbreak of SARS-CoV-2 Omicron Lineages, Immune Escape, and Vaccine Effectivity. J. Med. Virol. 2023, 95, e28138. [Google Scholar] [CrossRef] [PubMed]
- Pather, S.; Madhi, S.A.; Cowling, B.J.; Moss, P.; Kamil, J.P.; Ciesek, S.; Muik, A.; Türeci, Ö. SARS-CoV-2 Omicron Variants: Burden of Disease, Impact on Vaccine Effectiveness and Need for Variant-Adapted Vaccines. Front. Immunol. 2023, 14, 1130539. [Google Scholar] [CrossRef]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 Escape Antibodies Elicited by Omicron Infection. Nature 2022, 608, 593–602. [Google Scholar] [CrossRef]
- Flisiak, R.; Rzymski, P.; Zarębska-Michaluk, D.; Ciechanowski, P.; Dobrowolska, K.; Rogalska, M.; Jaroszewicz, J.; Szymanek-Pasternak, A.; Rorat, M.; Kozielewicz, D.; et al. Variability in the Clinical Course of COVID-19 in a Retrospective Analysis of a Large Real-World Database. Viruses 2023, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- Seyler, L.; Van Nedervelde, E.; De Cock, D.; Mann, C.; Pien, K.; Allard, S.D.; Demuyser, T. Surfing the Waves: Differences in Hospitalised COVID-19 Patients across 4 Variant Waves in a Belgian University Hospital. Viruses 2023, 15, 618. [Google Scholar] [CrossRef] [PubMed]
- WHO Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. Available online: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (accessed on 7 June 2023).
- Rzymski, P.; Pokorska-Śpiewak, M.; Jackowska, T.; Kuchar, E.; Nitsch-Osuch, A.; Pawłowska, M.; Babicki, M.; Jaroszewicz, J.; Szenborn, L.; Wysocki, J.; et al. Key Essentials during the Transition from the Acute Phase of the COVID-19 Pandemic. Preprints 2023, 2023081245. [Google Scholar] [CrossRef]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Parczewski, M.; Pawłowska, M.; Piekarska, A.; Simon, K.; et al. Management of SARS-CoV-2 Infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of April 26, 2021. Pol. Arch. Intern. Med. 2021, 131, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Parczewski, M.; Pawłowska, M.; Piekarska, A.; Simon, K.; et al. Management of SARS-CoV-2 Infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of February 23, 2022. Pol. Arch. Intern. Med. 2022, 132, 16230. [Google Scholar] [CrossRef]
- Genomic Epidemiology of SARS-CoV-2 with Subsampling Focused on Europe Since Pandemic Start. Available online: https://nextstrain.org/ncov/gisaid/europe/ (accessed on 25 June 2023).
- Flisiak, R.; Zarębska-Michaluk, D.; Rogalska, M.; Kryńska, J.A.; Kowalska, J.; Dutkiewicz, E.; Dobrowolska, K.; Jaroszewicz, J.; Moniuszko-Malinowska, A.; Rorat, M.; et al. Real-World Experience with Molnupiravir during the Period of SARS-CoV-2 Omicron Variant Dominance. Pharmacol. Rep. 2022, 74, 1279–1285. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney Disease Is Associated with In-Hospital Death of Patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef]
- Manathunga, S.S.; Abeyagunawardena, I.A.; Dharmaratne, S.D. A Comparison of Transmissibility of SARS-CoV-2 Variants of Concern. Virol. J. 2023, 20, 59. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Ayoub, H.H.; Coyle, P.; Tang, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Hasan, M.R.; Al-Kanaani, Z.; Al-Kuwari, E.; et al. Protection of Omicron Sub-Lineage Infection against Reinfection with Another Omicron Sub-Lineage. Nat. Commun. 2022, 13, 4675. [Google Scholar] [CrossRef]
- Modes, M.E.; Directo, M.P.; Melgar, M.; Johnson, L.R.; Yang, H.; Chaudhary, P.; Bartolini, S.; Kho, N.; Noble, P.W.; Isonaka, S.; et al. Clinical Characteristics and Outcomes Among Adults Hospitalized with Laboratory-Confirmed SARS-CoV-2 Infection During Periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) Variant Predominance—One Hospital, California, July 15–September 23, 2021, and December 21, 2021–January 27, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 217–223. [Google Scholar] [CrossRef]
- Mondi, A.; Mastrorosa, I.; Piselli, P.; Cimaglia, C.; Matusali, G.; Carletti, F.; Giannico, G.; Milozzi, E.; Biliotti, E.; Di Bari, S.; et al. Evolution of SARS-CoV-2 Variants of Concern over a Period of Delta and Omicron Cocirculation, among Patients Hospitalized for COVID-19 in an Italian Reference Hospital: Impact on Clinical Outcomes. J. Med. Virol. 2023, 95, e28831. [Google Scholar] [CrossRef] [PubMed]
- Karyakarte, R.P.; Das, R.; Dudhate, S.; Agarasen, J.; Pillai, P.; Chandankhede, P.M.; Labhshetwar, R.S.; Gadiyal, Y.; Rajmane, M.V.; Kulkarni, P.P.; et al. Clinical Characteristics and Outcomes of Laboratory-Confirmed SARS-CoV-2 Cases Infected with Omicron Subvariants and the XBB Recombinant Variant. Cureus 2023, 15, e35261. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Hong, V.X.; Patel, M.M.; Kahn, R.; Lipsitch, M.; Tartof, S.Y. Clinical Outcomes Associated with SARS-CoV-2 Omicron (B.1.1.529) Variant and BA.1/BA.1.1 or BA.2 Subvariant Infection in Southern California. Nat. Med. 2022, 28, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, J.; Yu, L.; Sui, X.; Wu, Q. Omicron Subvariant BA.5 Is Highly Contagious but Containable: Successful Experience from Macau. Front. Public. Health 2022, 10, 1029171. [Google Scholar] [CrossRef]
- Wolter, N.; Jassat, W.; DATCOV-Gen Author Group; von Gottberg, A.; Cohen, C. Clinical Severity of Omicron Lineage BA.2 Infection Compared with BA.1 Infection in South Africa. Lancet 2022, 400, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Groza, C.; Totschnig, D.; Wenisch, C.; Atamaniuk, J.; Zoufaly, A. A Retrospective Analysis of Clinical Features of Patients Hospitalized with SARS-CoV-2 Omicron Variants BA.1 and BA.2. Sci. Rep. 2023, 13, 7896. [Google Scholar] [CrossRef]
- Russell, S.L.; Klaver, B.R.A.; Harrigan, S.P.; Kamelian, K.; Tyson, J.; Hoang, L.; Taylor, M.; Sander, B.; Mishra, S.; Prystajecky, N.; et al. Clinical Severity of Omicron Subvariants BA.1, BA.2, and BA.5 in a Population-Based Cohort Study in British Columbia, Canada. J. Med. Virol. 2023, 95, e28423. [Google Scholar] [CrossRef]
- Morris, C.P.; Eldesouki, R.E.; Sachithanandham, J.; Fall, A.; Norton, J.M.; Abdullah, O.; Gallagher, N.; Li, M.; Pekosz, A.; Klein, E.Y.; et al. Omicron Subvariants: Clinical, Laboratory, and Cell Culture Characterization. Clin. Infect. Dis. 2023, 76, 1276–1284. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Bonato, M.; Cinque, P. Smell and Taste Disorders in COVID-19: From Pathogenesis to Clinical Features and Outcomes. Neurosci. Lett. 2021, 748, 135694. [Google Scholar] [CrossRef]
- Hornuss, D.; Lange, B.; Schröter, N.; Rieg, S.; Kern, W.V.; Wagner, D. Anosmia in COVID-19 Patients. Clin. Microbiol. Infect. 2020, 26, 1426–1427. [Google Scholar] [CrossRef]
- Nakakubo, S.; Kishida, N.; Okuda, K.; Kamada, K.; Iwama, M.; Suzuki, M.; Yokota, I.; Ito, Y.M.; Nasuhara, Y.; Boucher, R.C.; et al. Associations of COVID-19 Symptoms with Omicron Subvariants BA.2 and BA.5, Host Status, and Clinical Outcomes: A Registry-Based Observational Study in Sapporo, Japan. medRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Lee, J.H.; Kim, B.-N. Chest CT Findings in Hospitalized Patients with SARS-CoV-2: Delta versus Omicron Variants. Radiology 2023, 306, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, M.T.; Watson, R.A.; Saujani, S.J.; Kong, M.; Xie, C.; Peschl, H.; Wing, L.; MacLeod, F.K.; Shine, B.; Talbot, N.P.; et al. Reduction in Chest CT Severity and Improved Hospital Outcomes in SARS-CoV-2 Omicron Compared with Delta Variant Infection. Radiology 2023, 306, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Duong, B.V.; Larpruenrudee, P.; Fang, T.; Hossain, S.I.; Saha, S.C.; Gu, Y.; Islam, M.S. Is the SARS CoV-2 Omicron Variant Deadlier and More Transmissible Than Delta Variant? Int. J. Environ. Res. Public. Health 2022, 19, 4586. [Google Scholar] [CrossRef]
- Hansen, C.H.; Friis, N.U.; Bager, P.; Stegger, M.; Fonager, J.; Fomsgaard, A.; Gram, M.A.; Christiansen, L.E.; Ethelberg, S.; Legarth, R.; et al. Risk of Reinfection, Vaccine Protection, and Severity of Infection with the BA.5 Omicron Subvariant: A Nation-Wide Population-Based Study in Denmark. Lancet Infect. Dis. 2023, 23, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.-A.; Morden, E.; Rousseau, P.; Arendse, J.; Bam, J.-L.; Boloko, L.; Cloete, K.; Cohen, C.; Chetty, N.; Dane, P.; et al. Outcomes of Laboratory-Confirmed SARS-CoV-2 Infection during Resurgence Driven by Omicron Lineages BA.4 and BA.5 Compared with Previous Waves in the Western Cape Province, South Africa. Int. J. Infect. Dis. 2023, 127, 63–68. [Google Scholar] [CrossRef]
- Pascall, D.J.; Vink, E.; Blacow, R.; Bulteel, N.; Campbell, A.; Campbell, R.; Clifford, S.; Davis, C.; da Silva Filipe, A.; El Sakka, N.; et al. Directions of Change in Intrinsic Case Severity across Successive SARS-CoV-2 Variant Waves Have Been Inconsistent. J. Infect. 2023, 87, 128–135. [Google Scholar] [CrossRef]
- Kimura, I.; Yamasoba, D.; Tamura, T.; Nao, N.; Suzuki, T.; Oda, Y.; Mitoma, S.; Ito, J.; Nasser, H.; Zahradnik, J.; et al. Virological Characteristics of the SARS-CoV-2 Omicron BA.2 Subvariants, Including BA.4 and BA.5. Cell 2022, 185, 3992–4007.e16. [Google Scholar] [CrossRef]
- Xia, S.; Wang, L.; Jiao, F.; Yu, X.; Xu, W.; Huang, Z.; Li, X.; Wang, Q.; Zhu, Y.; Man, Q.; et al. SARS-CoV-2 Omicron Subvariants Exhibit Distinct Fusogenicity, but Similar Sensitivity, to Pan-CoV Fusion Inhibitors. Emerg. Microbes Infect. 2023, 12, 2178241. [Google Scholar] [CrossRef]
- Xia, S.; Jiao, F.; Wang, L.; Yu, X.; Lu, T.; Fu, Y.; Huang, Z.; Li, X.; Huang, J.; Wang, Q.; et al. SARS-CoV-2 Omicron XBB Subvariants Exhibit Enhanced Fusogenicity and Substantial Immune Evasion in Elderly Population, but High Sensitivity to Pan-Coronavirus Fusion Inhibitors. J. Med. Virol. 2023, 95, e28641. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Bruel, T.; Staropoli, I.; Guivel-Benhassine, F.; Porrot, F.; Maes, P.; Grzelak, L.; Prot, M.; Mougari, S.; Planchais, C.; et al. Resistance of Omicron Subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to Neutralizing Antibodies. bioRxiv 2022. [Google Scholar] [CrossRef]
- Takashita, E.; Yamayoshi, S.; Simon, V.; van Bakel, H.; Sordillo, E.M.; Pekosz, A.; Fukushi, S.; Suzuki, T.; Maeda, K.; Halfmann, P.; et al. Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants. N. Engl. J. Med. 2022, 387, 468–470. [Google Scholar] [CrossRef]
- Imai, M.; Ito, M.; Kiso, M.; Yamayoshi, S.; Uraki, R.; Fukushi, S.; Watanabe, S.; Suzuki, T.; Maeda, K.; Sakai-Tagawa, Y.; et al. Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB. N. Engl. J. Med. 2023, 388, 89–91. [Google Scholar] [CrossRef]
- Bergeri, I.; Whelan, M.G.; Ware, H.; Subissi, L.; Nardone, A.; Lewis, H.C.; Li, Z.; Ma, X.; Valenciano, M.; Cheng, B.; et al. Global SARS-CoV-2 Seroprevalence from January 2020 to April 2022: A Systematic Review and Meta-Analysis of Standardized Population-Based Studies. PLoS Med. 2022, 19, e1004107. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, J.R.C.; van Schalkwyk, C.; Govender, N.; von Gottberg, A.; Cohen, C.; Groome, M.J.; Dushoff, J.; Mlisana, K.; Moultrie, H. Increased Risk of SARS-CoV-2 Reinfection Associated with Emergence of Omicron in South Africa. Science 2022, 376, eabn4947. [Google Scholar] [CrossRef] [PubMed]


| Early Omicron (n = 965) | Late Omicron (n = 897) | p-Value | |
|---|---|---|---|
| The average number of patients per month, n | 161 | 90 | - |
| Demographic characteristics | |||
| BMI, x ±SD | 27.0 ± 5.5 | 27.1 ± 5.5 | U = 278,693, z = 0.15, p = 0.884 |
| Gender, females/males, n (%) | 494/471 (51.2/48.8) | 472/425 (52.6/47.4) | χ2 = 0.3, p = 0.538 |
| Age (years), mean ±SD | 68.7 ± 18.2 | 73.7 ± 14.8 | U = 370,909, z = −5.30, p < 0.001 |
| <20 years, n (%) | 1 (0.1) | 2 (0.2) | p = 0.611 |
| 20–40 years, n (%) | 106 (11.0) | 36 (4.0) | χ2 = 32.1, p < 0.001 |
| 40–60 years, n (%) | 142 (14.7) | 89 (9.9) | χ2 = 9.8, p = 0.002 |
| 60–80 years, n (%) | 416 (43.2) | 452 (50.4) | χ2 = 9.9, p = 0.002 |
| >80 years, n (%) | 299 (31.0) | 318 (35.5) | χ2 = 4.2, p = 0.04 |
| Comorbidities | |||
| Any comorbidities | 889 (92.1) | 828 (92.3) | χ2 = 0.02, p = 0.883 |
| Hypertension | 552 (57.2) | 591 (65.9) | χ2 = 14.8, p = 0.002 |
| Myocardial ischemic disease | 200 (20.7) | 243 (27.1) | χ2 = 10.4, p = 0.013 |
| Other cardiovascular diseases | 310 (32.1) | 271 (30.2) | χ2 = 0.79, p = 0.373 |
| Chronic obstructive pulmonary disease | 74 (7.7) | 71 (7.9) | χ2 = 0.04, p = 0.843 |
| Other respiratory diseases | 114 (11.8) | 80 (8.9) | χ2 = 4.3, p = 0.039 |
| Diabetes | 239 (24.8) | 233 (26.0) | χ2 = 0.36, p = 0.549 |
| Other metabolic diseases | 144 (14.9) | 137 (15.3) | χ2 = 0.045, p = 0.833 |
| Cancers | 150 (15.5) | 155 (17.3) | χ2 = 1.0, p = 0.312 |
| Stroke | 95 (9.8) | 92 (10.3) | χ2 = 0.09, p = 0.768 |
| Early Omicron (n = 965) | Late Omicron (n = 897) | p-Value | |
|---|---|---|---|
| Symptoms of the disease | |||
| Cough, n (%) | 475 (49.2) | 441 (49.2) | χ2 = 0.001, p = 0.980 |
| Fever, n (%) | 470 (48.7) | 506 (56.4) | χ2 = 9.6, p = 0.002 |
| Dyspnea, n (%) | 350 (36.3) | 307 (34.2) | χ2 = 0.851, p = 0.356 |
| Disturbances of smell and/or taste, n (%) | 25 (2.6) | 6 (0.7) | p = 0.002 |
| Diarrhea, n (%) | 101 (10.5) | 72 (8.0) | χ2 = 3.2, p = 0.07 |
| Headaches, n (%) | 89 (9.2) | 99 (11.0) | χ2 = 1.7, p = 0.194 |
| Nausea, n (%) | 71 (7.4) | 69 (7.7) | χ2 = 0.07, p = 0.784 |
| Vomiting, n (%) | 66 (6.8) | 69 (7.7) | χ2 = 0.503, p = 0.478 |
| Fatigue, n (%) | 329 (34.1) | 351 (39.1) | χ2 = 5.1, p = 0.02 |
| Respiratory function on admission to the hospital | |||
| SpO2 (%), mean ± SD | 92.1 ± 5.6 | 92.5 ± 5.8 | U = 376,903, z = −2.35, p < 0.001 |
| Asymptomatic, n (%) | 49 (5.1) | 19 (2.1) | χ2 = 11.6, p < 0.001 |
| Symptomatic stable, SpO2 > 95%, n (%) | 288 (29.8) | 315 (35.1) | χ2 = 5.9, p = 0.015 |
| Symptomatic unstable, SpO2 90–95%, n (%) | 306 (31.7) | 297 (33.1) | χ2 = 0.41, p = 0.519 |
| Symptomatic unstable, SpO2 ≤ 90%, n (%) | 293 (30.4) | 213 (23.7) | χ2 = 4.3, p = 0.04 |
| ARDS, n (%) | 10 (1.0) | 6 (0.7) | p = 0.457 |
| Unknown, n (%) | 19 (2.0) | 47 (5.2) | χ2 = 14.5, p < 0.001 |
| Lung changes on imaging | |||
| Examinations done, n (%) | 823 (85.3) | 799 (89.1) | χ2 = 6.0, p = 0.015 |
| Changes detected by any method, n (% of done) | 484 (58.8) | 341 (42.7) | χ2 = 27.8, p < 0.001 |
| Changes detected in X-ray, n (% of done) | 131 (15.9) | 108 (13.5) | χ2 = 0.98, p = 0.322 |
| Changes detected by CT n (% of done) | 366 (44.5) | 241 (30.2) | χ2 = 25.9, p < 0.001 |
| Changes detected by ultrasound, n (% of done) | 1 (0.1) | 1 (0.1) | p = 1.0 |
| Treatment administered | |||
| Heparin, n (%) | 558 (57.8) | 511 (57.0) | χ2 = 0.139, p = 0.709 |
| Remdesivir, n (%) | 276 (28.6) | 392 (43.7) | χ2 = 46.1, p < 0.001 |
| Molnupiravir, n (%) | 233 (24.1) | 103 (11.5) | χ2 = 50.4, p < 0.001 |
| Nirmatrelvir/ritonavir, n (%) | 0 (0.0) | 66 (7.4) | p < 0.001 |
| Casirivimab, n (%) | 21 (2.2) | 0 (0.0) | p < 0.001 |
| Convalescent plasma, n (%) | 4 (0.4) | 0 (0.0) | p = 0.126 |
| Dexamethasone, n (%) | 305 (31.6) | 190 (21.2) | χ2 = 25.9, p < 0.001 |
| Tocilizumab, n (%) | 89 (9.2) | 23 (2.6) | χ2 = 36.5, p < 0.001 |
| Baricitinib, n (%) | 29 (3.0) | 1 (0.1) | p < 0.001 |
| Azithromycin, n (%) | 3 (0.3) | 5 (0.6) | p = 0.493 |
| Clinical improvement (at least 2 points decrease from baseline in the ordinal scale) | |||
| 7 days, n (%) | 180 (18.8) | 294 (34.4) | χ2 = 48.9, p < 0.001 |
| 14 days, n (%) | 612 (63.8) | 641 (75.1) | χ2 = 13.7, p = 0.002 |
| 21 days, n (%) | 735 (76.6) | 709 (83.0) | χ2 = 2.2, p = 0.137 |
| 28 days, n (%) | 779 (81.2) | 741 (86.8) | χ2 = 1.1, p = 0.294 |
| Number of Patients n | Need for Oxygen Therapy n (%) | Need for Mechanical Ventilation n (%) | Death n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| EO | LO | EO | LO | EO | LO | EO | LO | |
| All patients | 965 | 897 | 457 (47.4) | 344 (38.4) | 29 (3.0) | 13 (1.5) | 134 (13.9) | 80 (8.9) |
| χ2 = 15.4, p < 0.001 | χ2 = 5.1, p = 0.02 | χ2 = 11.3, p < 0.001 | ||||||
| Age >60 years (60+) | 716 | 775 | 395 (55.2) | 331 (42.7) | 24 (3.4) | 12 (1.5) | 124 (17.3) | 77 (9.9) |
| χ2 = 23.1, p < 0.001 | χ2 = 3.4, p = 0.06 | χ2 = 17.4, p < 0.001 | ||||||
| Imaging changes (IC) | 484 | 556 | 318 (65.7) | 198 (58.1) | 23 (4.8) | 11 (3.2) | 78 (16.1) | 54 (15.8) |
| χ2 = 93.7, p < 0.001 | χ2 = 6.3, p = 0.01 | χ2 = 9.6, p = 0.002 | ||||||
| Comorbidities (CM) | 889 | 828 | 432 (48.6) | 336 (40.6) | 26 (2.9) | 13 (1.6) | 129 (14.5) | 78 (9.4) |
| χ2 = 11.1, p < 0.001 | χ2 = 3.5, p = 0.06 | χ2 = 10.5, p = 0.001 | ||||||
| Accumulation of risk factors | ||||||||
| 60+/IC/CM | 383 | 307 | 270 (70.5) | 188 (61.2) | 18 (4.7) | 10 (3.3) | 71 (18.5) | 52 (17.7) |
| χ2 = 6.5, p = 0.01 | χ2 = 0.91, p = 0.340 | χ2 = 0.29, p = 0.585 | ||||||
| 60+/IC/CM/SpO2 < 95% | 306 | 241 | 252 (82.4) | 181 (75.1) | 15 (4.9) | 10 (4.2) | 59 (19.3) | 47 (19.5) |
| χ2 = 4.3, p = 0.04 | χ2 = 0.17, p = 0.675 | χ2 = 0.004, p = 0.948 | ||||||
| 60+/IC/CM/SpO2 < 90% | 165 | 126 | 155 (93.9) | 123 (97.6) | 11 (6.9) | 10 (7.9) | 40 (24.2) | 38 (30.2) |
| p = 0.160 | χ2 = 0.17, p = 0.678 | χ2 = 1.27, p = 0.259 | ||||||
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Predicted outcome: death | |||
| Age 60+ | 0.93 | 0.59–1.45 | 0.741 |
| BMI > 30 m2/kg | 0.88 | 0.59–1.29 | 0.502 |
| Male sex | 0.99 | 0.70–1.39 | 0.959 |
| Imaging changes | 1.08 | 0.76–1.53 | 0.661 |
| Comorbidities | 2.36 | 1.19–6.20 | 0.0317 |
| SpO2 < 90% | 2.04 | 1.40–2.96 | 0.0002 |
| Late Omicron phase | 0.55 | 0.39–0.79 | 0.0014 |
| Predicted outcome: mechanical ventilation | |||
| Age 60+ | 0.76 | 0.31–1.90 | 0.562 |
| BMI > 30 m2/kg | 0.82 | 0.36–1.86 | 0.636 |
| Male sex | 0.80 | 0.40–1.64 | 0.545 |
| Imaging changes | 2.21 | 1.05–4.67 | 0.038 |
| Comorbidities | 3.45 | 0.43–27.71 | 0.244 |
| SpO2 < 90% | 1.44 | 1.12–3.23 | 0.037 |
| Late Omicron phase | 0.52 | 0.23–1.14 | 0.103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flisiak, R.; Zarębska-Michaluk, D.; Dobrowolska, K.; Rorat, M.; Rogalska, M.; Kryńska, J.A.; Moniuszko-Malinowska, A.; Czupryna, P.; Kozielewicz, D.; Jaroszewicz, J.; et al. Change in the Clinical Picture of Hospitalized Patients with COVID-19 between the Early and Late Period of Dominance of the Omicron SARS-CoV-2 Variant. J. Clin. Med. 2023, 12, 5572. https://doi.org/10.3390/jcm12175572
Flisiak R, Zarębska-Michaluk D, Dobrowolska K, Rorat M, Rogalska M, Kryńska JA, Moniuszko-Malinowska A, Czupryna P, Kozielewicz D, Jaroszewicz J, et al. Change in the Clinical Picture of Hospitalized Patients with COVID-19 between the Early and Late Period of Dominance of the Omicron SARS-CoV-2 Variant. Journal of Clinical Medicine. 2023; 12(17):5572. https://doi.org/10.3390/jcm12175572
Chicago/Turabian StyleFlisiak, Robert, Dorota Zarębska-Michaluk, Krystyna Dobrowolska, Marta Rorat, Magdalena Rogalska, Justyna Anna Kryńska, Anna Moniuszko-Malinowska, Piotr Czupryna, Dorota Kozielewicz, Jerzy Jaroszewicz, and et al. 2023. "Change in the Clinical Picture of Hospitalized Patients with COVID-19 between the Early and Late Period of Dominance of the Omicron SARS-CoV-2 Variant" Journal of Clinical Medicine 12, no. 17: 5572. https://doi.org/10.3390/jcm12175572
APA StyleFlisiak, R., Zarębska-Michaluk, D., Dobrowolska, K., Rorat, M., Rogalska, M., Kryńska, J. A., Moniuszko-Malinowska, A., Czupryna, P., Kozielewicz, D., Jaroszewicz, J., Sikorska, K., Bednarska, A., Piekarska, A., & Rzymski, P. (2023). Change in the Clinical Picture of Hospitalized Patients with COVID-19 between the Early and Late Period of Dominance of the Omicron SARS-CoV-2 Variant. Journal of Clinical Medicine, 12(17), 5572. https://doi.org/10.3390/jcm12175572