One-Year Mortality in Patients Undergoing an Implantable Cardioverter Defibrillator or Cardiac Resynchronization Therapy Pulse Generator Replacement: Identifying Patients at Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Endpoints
2.3. Statistical Analysis
2.4. Ethical Statement
3. Results
3.1. Study Population
3.2. Stratification According to Type of Device
3.3. Pharmacotherapy
3.4. One-Year Mortality after Pulse Generator Replacement
3.5. Validation Risk Score of Kraaier et al.
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 esc guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Klein, H.; Levine, J.H.; Saksena, S.; Waldo, A.L.; Wilber, D.; et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N. Engl. J. Med. 1996, 335, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Kadish, A.; Dyer, A.; Daubert, J.P.; Quigg, R.; Estes, N.A.; Anderson, K.P.; Calkins, H.; Hoch, D.; Goldberger, J.; Shalaby, A.; et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N. Engl. J. Med. 2004, 350, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005, 352, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Jukema, J.W.; Timal, R.J.; Rotmans, J.I.; Hensen, L.C.R.; Buiten, M.S.; de Bie, M.K.; Putter, H.; Zwinderman, A.H.; van Erven, L.; Krol-van Straaten, M.J.; et al. Prophylactic use of implantable cardioverter-defibrillators in the prevention of sudden cardiac death in dialysis patients the prospective, randomized, controlled icd2 trial. Circulation 2019, 139, 2628–2638. [Google Scholar] [CrossRef]
- Yusuf, S.; Pitt, B.; Davis, C.E.; Hood, W.B., Jr.; Cohn, J.N. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N. Engl. J. Med. 1992, 327, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Hjalmarson, A.; Goldstein, S.; Fagerberg, B.; Wedel, H.; Waagstein, F.; Kjekshus, J.; Wikstrand, J.; El Allaf, D.; Vítovec, J.; Aldershvile, J.; et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: The metoprolol cr/xl randomized intervention trial in congestive heart failure (merit-hf). Merit-hf study group. JAMA 2000, 283, 1295–1302. [Google Scholar] [CrossRef]
- Cleland, J.G.; Abraham, W.T.; Linde, C.; Gold, M.R.; Young, J.B.; Daubert, J.C.; Sherfesee, L.; Wells, G.A.; Tang, A.S. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur. Heart J. 2013, 34, 3547–3556. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Køber, L.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbæk, L.; Korup, E.; Jensen, G.; Hildebrandt, P.; Steffensen, F.H.; Bruun, N.E.; et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N. Engl. J. Med. 2016, 375, 1221–1230. [Google Scholar] [CrossRef]
- Disertori, M.; Quintarelli, S.; Mazzola, S.; Favalli, V.; Narula, N.; Arbustini, E. The need to modify patient selection to improve the benefits of implantable cardioverter-defibrillator for primary prevention of sudden death in non-ischaemic dilated cardiomyopathy. Europace 2013, 15, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Steckman, D.A.; Varosy, P.D.; Parzynski, C.S.; Masoudi, F.A.; Curtis, J.P.; Sauer, W.H.; Nguyen, D.T. In-hospital complications associated with reoperations of implantable cardioverter defibrillators. Am. J. Cardiol. 2014, 114, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Borleffs, C.J.W.; Thijssen, J.; de Bie, M.K.; van Rees, J.B.; van Welsenes, G.H.; van Erven, L.; Bax, J.J.; Cannegieter, S.C.; Schalij, M.J. Recurrent implantable cardioverter-defibrillator replacement is associated with an increasing risk of pocket-related complications. Pace 2010, 33, 1013–1019. [Google Scholar] [CrossRef]
- Junttila, M.J.; Pelli, A.; Kenttä, T.V.; Friede, T.; Willems, R.; Bergau, L.; Malik, M.; Vandenberk, B.; Vos, M.A.; Schmidt, G.; et al. Appropriate shocks and mortality in patients with versus without diabetes with prophylactic implantable cardioverter defibrillators. Diabetes Care 2020, 43, 196–200. [Google Scholar] [CrossRef]
- Zabel, M.; Willems, R.; Lubinski, A.; Bauer, A.; Brugada, J.; Conen, D.; Flevari, P.; Hasenfuß, G.; Svetlosak, M.; Huikuri, H.V.; et al. Clinical effectiveness of primary prevention implantable cardioverter-defibrillators: Results of the eu-cert-icd controlled multicentre cohort study. Eur. Heart J. 2020, 41, 3437–3447. [Google Scholar] [CrossRef]
- Savelieva, I.; Fumagalli, S.; Kenny, R.A.; Anker, S.; Benetos, A.; Boriani, G.; Bunch, J.; Dagres, N.; Dubner, S.; Fauchier, L.; et al. Ehra expert consensus document on the management of arrhythmias in frailty syndrome, endorsed by the heart rhythm society (hrs), asia pacific heart rhythm society (aphrs), latin america heart rhythm society (lahrs), and cardiac arrhythmia society of southern africa (cassa). Europace 2023, 25, 1249–1276. [Google Scholar] [CrossRef]
- Kraaier, K.; Scholten, M.F.; Tijssen, J.G.; Theuns, D.A.; Jordaens, L.J.; Wilde, A.A.; van Dessel, P.F. Early mortality in prophylactic implantable cardioverter-defibrillator recipients: Development and validation of a clinical risk score. Europace 2014, 16, 40–46. [Google Scholar] [CrossRef]
- Mond, H.G.; Proclemer, A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: Calendar year 2009—A world society of arrhythmia’s project. Pacing Clin. Electrophysiol. 2011, 34, 1013–1027. [Google Scholar] [CrossRef]
- Van Volksgezondheid, M.; en Sport, W. Verbetersignalement zinnige zorg implanteerbare cardioverter-defibrillator (icd). Zorginstituut Ned. 2023, 81. [Google Scholar]
- Kramer, D.B.; Kennedy, K.F.; Spertus, J.A.; Normand, S.L.; Noseworthy, P.A.; Buxton, A.E.; Josephson, M.E.; Zimetbaum, P.J.; Mitchell, S.L.; Reynolds, M.R. Mortality risk following replacement implantable cardioverter-defibrillator implantation at end of battery life: Results from the ncdr. Heart Rhythm 2014, 11, 216–221. [Google Scholar] [CrossRef]
- Demarchi, A.; Cornara, S.; Sanzo, A.; Savastano, S.; Petracci, B.; Vicentini, A.; Pontillo, L.; Baldi, E.; Frigerio, L.; Astuti, M.; et al. Incidence of ventricular arrhythmias and 1-year predictors of mortality in patients treated with implantable cardioverter-defibrillator undergoing generator replacement. J. Am. Heart Assoc. 2021, 10, e018090. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 esc guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, D.; Egorova, A.D.; Schalij, M.J.; Spierenburg, H.A.M.; Verbunt, R.A.M.; van Erven, L. The development of a decision aid for shared decision making in the dutch implantable cardioverter defibrillator patient population: A novel approach to patient education. Front. Cardiovasc. Med. 2022, 9, 946404. [Google Scholar] [CrossRef] [PubMed]
- Duray, G.Z.; Schmitt, J.; Richter, S.; Israel, C.W.; Hohnloser, S.H. Arrhythmic death in implantable cardioverter defibrillator patients: A long-term study over a 10 year implantation period. Europace 2009, 11, 1462–1468. [Google Scholar] [CrossRef]
- van Rees, J.B.; Borleffs, C.J.; van Welsenes, G.H.; van der Velde, E.T.; Bax, J.J.; van Erven, L.; Putter, H.; van der Bom, J.G.; Schalij, M.J. Clinical prediction model for death prior to appropriate therapy in primary prevention implantable cardioverter defibrillator patients with ischaemic heart disease: The fades risk score. Heart 2012, 98, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Theuns, D.; Niazi, K.; Schaer, B.A.; Sticherling, C.; Yap, S.C.; Caliskan, K. Reassessment of clinical variables in cardiac resynchronization defibrillator patients at the time of first replacement: Death after replacement of crt (darc) score. J. Cardiovasc. Electrophysiol. 2021, 32, 1687–1694. [Google Scholar] [CrossRef]
- Jędrzejczyk-Patej, E.; Mazurek, M.; Kotalczyk, A.; Kowalska, W.; Konieczny-Kozielska, A.; Kozielski, J.; Podolecki, T.; Szulik, M.; Sokal, A.; Kowalski, O.; et al. Upgrade from implantable cardioverter-defibrillator vs. De novo implantation of cardiac resynchronization therapy: Long-term outcomes. Europace 2021, 23, 113–122. [Google Scholar] [CrossRef]
- Iuliano, S.; Fisher, S.G.; Karasik, P.E.; Fletcher, R.D.; Singh, S.N. Qrs duration and mortality in patients with congestive heart failure. Am. Heart J. 2002, 143, 1085–1091. [Google Scholar] [CrossRef]
- Baldasseroni, S.; Gentile, A.; Gorini, M.; Marchionni, N.; Marini, M.; Masotti, G.; Porcu, M.; Maggioni, A.P. Intraventricular conduction defects in patients with congestive heart failure: Left but not right bundle branch block is an independent predictor of prognosis. A report from the italian network on congestive heart failure (in-chf database). Ital. Heart J. 2003, 4, 607–613. [Google Scholar] [CrossRef]
- Mascia, G.; Perini, A.P.; Cartei, S.; Binazzi, B.; Gigliotti, F.; Solimene, F.; Mascioli, G.; Giaccardi, M. Sleep-disordered breathing and effectiveness of cardiac resynchronization therapy in heart failure patients: Gender differences? Sleep Med. 2019, 64, 106–111. [Google Scholar] [CrossRef]
- Assa, S.; Vernooy, K.; van Stipdonk, A.M.W. Cardiovascular implantable electronic devices enabled remote heart failure monitoring; what we have learned and where to go next. J. Cardiovasc. Dev. Dis. 2023, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Simantirakis, E.N.; Schiza, S.E.; Siafakas, N.S.; Vardas, P.E. Sleep-disordered breathing in heart failure and the effect of cardiac resynchronization therapy. Europace 2008, 10, 1029–1033. [Google Scholar] [CrossRef]
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A contemporary appraisal of the heart failure epidemic in olmsted county, minnesota, 2000 to 2010. JAMA Intern. Med. 2015, 175, 996–1004. [Google Scholar] [CrossRef]
- Yilmaz, D.; Egorova, A.D.; Schalij, M.J.; van Erven, L. Implantable cardioverter-defibrillators and the older patient: The dutch clinical practice. Eur. J. Cardiovasc. Nurs. 2022, 21, 169–173. [Google Scholar] [CrossRef]
- Yilmaz, D.; van der Heijden, A.C.; Thijssen, J.; Schalij, M.J.; van Erven, L. Patients with an icd remain at risk for painful shocks in last moments of life. J. Am. Coll. Cardiol. 2017, 70, 1681–1682. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Potpara, T.S.; Boveda, S.; Deharo, J.C.; Chen, J.; Dobreanu, D.; Fumagalli, S.; Lenarczyk, R.; Madrid, A.H.; Larsen, T.B.; et al. Patients’ knowledge and attitudes regarding living with implantable electronic devices: Results of a multicentre, multinational patient survey conducted by the european heart rhythm association. Europace 2018, 20, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, S.; Pieragnoli, P.; Haugaa, K.H.; Potpara, T.S.; Rasero, L.; Ramacciati, N.; Ricciardi, G.; Solimene, F.; Mascia, G.; Mascioli, G.; et al. The influence of age on the psychological profile of patients with cardiac implantable electronic devices: Results from the italian population in a multicenter study conducted by the european heart rhythm association. Aging Clin. Exp. Res. 2019, 31, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Elwyn, G.; Scholl, I.; Tietbohl, C.; Mann, M.; Edwards, A.G.; Clay, C.; Légaré, F.; van der Weijden, T.; Lewis, C.L.; Wexler, R.M.; et al. “Many miles to go …”: A systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med. Inform. Decis. Mak. 2013, 13 (Suppl. S2), S14. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 esc guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Frey, S.M.; Brenner, R.; Theuns, D.A.; Al-Shoaibi, N.; Crawley, R.J.; Ammann, P.; Sticherling, C.; Kühne, M.; Osswald, S.; Schaer, B. Follow-up of crt-d patients downgraded to crt-p at the time of generator exchange. Front. Cardiovasc. Med. 2023, 10, 1217523. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Theriault-Lauzier, P.; Birnie, D.; Redpath, C.; Golian, M.; Sadek, M.M.; Klein, A.; Ramirez, F.D.; Davis, D.R.; Nery, P.B.; et al. Should they stay, or should they go: Do we need to remove the old cardiac implantable electronic device if a new system is required on the contralateral side? Heart Rhythm O2 2022, 3, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.; Lupercio, F.; Ho, G.; Pollema, T.; Pretorius, V.; Birgersdotter-Green, U. Safety and efficacy of cardiovascular implantable electronic device extraction in elderly patients: A meta-analysis and systematic review. Heart Rhythm O2 2020, 1, 250–258. [Google Scholar] [CrossRef]
- Senes, J.; Mascia, G.; Bottoni, N.; Oddone, D.; Donateo, P.; Grimaldi, T.; Minneci, C.; Bertolozzi, I.; Brignole, M.; Puggioni, E.; et al. Is his-optimized superior to conventional cardiac resynchronization therapy in improving heart failure? Results from a propensity-matched study. Pacing Clin. Electrophysiol. 2021, 44, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Herweg, B.; Ellenbogen, K.A.; Gajek, J. His-optimized cardiac resynchronization therapy to maximize electrical resynchronization: A feasibility study. Circ. Arrhythm Electrophysiol. 2019, 12, e006934. [Google Scholar] [CrossRef]






| Total Cohort (n = 588) | ICD Exchange (n = 286) | CRT-D Exchange (n = 234) | CRT-D Upgrade (n = 68) | p-Value ICD vs. CRT-D | p-Value CRT Exchange vs. Upgrade | |
|---|---|---|---|---|---|---|
| Gender, male (%) | 453 (77) | 218 (76) | 181 (77) | 54 (79) | 0.75 | 0.51 |
| Age in years [Q1–Q3] | 69 [60–76] | 66 [54–75] | 70 [64–77] | 70 [63–76] | 0.00 | 0.78 |
| BMI in kg/m2 [Q1–Q3] | 26.5 [24.1–29.7] | 26.2 [23.8–29.6] | 26.9 [24.7–30.3] | 26.4 [24.0–30.0] | 0.13 | 0.97 |
| NYHA-class | 0.00 | 0.00 | ||||
| I, n (%) | 244 (41) | 168 (58) | 69 (29) | 7 (10) | ||
| II, n (%) | 236 (40) | 89 (31) | 114 (49) | 33 (49) | ||
| III, n (%) | 92 (16) | 24 (9) | 44 (19) | 24 (35) | ||
| IV, n (%) | 12 (2) | 3 (1) | 5 (2) | 4 (6) | ||
| Unavailable, n (%) | 4 (1) | 2 (1) | 2 (1) | 0 (0) | ||
| CIED indication | 0.00 | 0.02 | ||||
| Primary prevention, n (%) | 349 (59) | 146 (51) | 166 (71) | 37 (54) | ||
| Secondary prevention, n (%) | 239 (41) | 140 (49) | 68 (29) | 31 (46) | ||
| Underlying cardiac condition | 0.00 | 0.414 | ||||
| Ischemic cardiomyopathy, n (%) | 270 (46) | 121 (42) | 115 (49) | 34 (50) | ||
| Non-ischemic cardiomyopathy, n (%) | 242 (41) | 99 (35) | 112 (48) | 31 (46) | ||
| Congenital heart disease n (%) | 29 (5) | 23 (8) | 5 (2) | 1 (2) | ||
| Electrical heart disease n (%) | 47 (8) | 43 (15) | 2 (1) | 2 (3) | 0.11 | 0.15 |
| Cardiac history | ||||||
| Atrial fibrillation n (%) | 240 (41) | 93 (33) | 108 (46) | 39 (57) | 0.00 | 0.00 |
| Paroxysmal, n (%) | 155 (65 | 67 (72) | 59 (55) | 29 (74) | ||
| Longstanding/persistent, n (%) | 85 (35) | 26 (28) | 49 (45) | 10 (26) | ||
| PCI, n (%) | 183 (31) | 81 (28) | 70 (30) | 32 (47) | 0.01 | 0.00 |
| CABG, n (%) | 121 (21) | 49 (17) | 54 (23) | 18 (26) | 0.10 | 0.20 |
| Valve surgery, n (%) | 129 (22) | 41 (14) | 72 (31) | 16 (24) | 0.00 | 0.64 |
| Echocardiographic findings | ||||||
| LVEF | 0.00 | 0.00 | ||||
| Good, n (%) | 86 (15) | 70 (25) | 12 (5) | 4 (6) | ||
| Mildly reduced, n (%) | 215 (37) | 118 (42) | 87 (37) | 10 (15) | ||
| Moderately reduced, n (%) | 188 (32) | 77 (27) | 86 (37) | 25 (37) | ||
| Poor, n (%) | 96 (16) | 18 (6) | 50 (21) | 28 (42) | ||
| Aortic valve insufficiency (%) | 0.15 | 0.11 | ||||
| No or mild, n (%) | 535 (91) | 260 (91) | 217 (93) | 58 (85) | ||
| Moderate, n (%) | 50 (9) | 23 (8) | 18 (7) | 17 (25) | ||
| Severe, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Mitral valve regurgitation (%) | 0.00 | 0.00 | ||||
| No or mild, n (%) | 410 (70) | 226 (79) | 152 (65) | 35 (52) | ||
| Moderate, n (%) | 167 (29) | 59 (20) | 80 (34) | 28 (42) | ||
| Severe, n (%) | 8 (1) | 1 (1) | 3 (1) | 4 (6) | ||
| Tricuspid valve insufficiency (%) | 0.00 | 0.00 | ||||
| No or mild, n (%) | 413 (71) | 222 (78) | 156 (67) | 35 (52) | ||
| Moderate, n (%) | 164 (28) | 59 (21) | 77 (32) | 28 (42) | ||
| Severe, n (%) | 8 (1) | 2 (1) | 2 (1) | 4 (6) | ||
| sPAP in mm Hg, median [Q1–Q3] | 27 [21–34] | 26 [20–32] | 28 [21–35] | 30 [23–38] | 0.03 | 0.11 |
| Comorbidities and risk factors | ||||||
| Hypertension, n (%) | 255 (43) | 118 (41) | 110 (47) | 27 (40) | 0.39 | 0.60 |
| Diabetes mellitus, n (%) | 126 (20) | 40 (20) | 59 (25) | 17 (25) | 0.00 | 0.25 |
| COPD, n (%) | 110 (19) | 50 (18) | 45 (19) | 15 (22) | 0.64 | 0.40 |
| Renal function, n (%) eGFR in mL/min/1.73 m2 | 0.00 | 0.00 | ||||
| Normal, eGFR ≥ 60, n (%) | 350 (59) | 202 (71) | 121 (52) | 27 (40) | ||
| Moderately reduced, eGFR 30–59, n (%) | 173 (29) | 59 (21) | 82 (35) | 32 (47) | ||
| Severely reduced, eGFR 15–29, n (%) | 34 (6) | 10 (4) | 18 (8) | 6 (9) | ||
| Kidney failure, eGFR < 15, n (%) | 9 (2) | 2 (1) | 7 (3) | 0 (0) | ||
| Missing, n (%) | 22 (4) | 13 (4) | 6 (1) | 3 (4) | ||
| Hypercholesteremia, n (%) | 181 (31) | 69 (24) | 91 (39) | 21 (31) | 0.00 | 0.89 |
| CVA/TIA, n (%) | 68 (12) | 29 (10) | 26 (11) | 13 (19) | 0.09 | 0.04 |
| Gastro-intestinal disease, n (%) | 66 (11) | 24 (8) | 33 (14) | 9 (13) | 0.11 | 0.54 |
| History of malignancy, n (%) | 78 (13) | 34 (12) | 34 (15) | 10 (15) | 0.63 | 0.70 |
| PADIT-risk score | ||||||
| Low risk (0–4) | 154 (26) | 154 (54) | 0 (0) | 0 (0) | 0.00 | - |
| Intermediate risk (5 or 6) | 267 (45) | 125 (44) | 128 (55) | 14 (21) | 0.00 | 0.00 |
| High risk (≥7) | 167 (28) | 7 (3) | 14 (21) | 54 (79) | 0.00 | 0.00 |
| Laboratory findings | ||||||
| Creatinine in mmol/L, n [Q1–Q3] | 92 [77–118] | 86 [74–105] | 99 [80–133] | 110 [95–140] | 0.00 | 0.00 |
| eGFR in mL/min/1.73 m2, n [Q1–Q3] | 68 [48–86] | 77 [59–89] | 62 [40–80] | 55 [40–69] | 0.00 | 0.00 |
| Potassium in mmol/L, n [Q1–Q3] | 4.4 [4.2–4.7] | 4.4 [4.2–4.7] | 4.4 [4.2–4.7] | 4.5 [4.2–4.8] | 0.66 | 0.41 |
| Medication | Total Cohort (n = 588) | ICD Exchange (n = 286) | CRT-D Exchange (n = 234) | CRT-D Upgrade (n = 68) | p-Value ICD vs. CRT | p-Value CRT Exchange vs. Upgrade |
|---|---|---|---|---|---|---|
| Anti-arrhythmic agents (%) | ||||||
| Class I, n (%) | 16 (2) | 10 (4) | 3 (1) | 3 (4) | 0.19 | 0.35 |
| Class II, n (%) | 385 (66) | 172 (60) | 169 (72) | 44 (65) | 0.04 | 0.97 |
| Class III, n (%) | 190 (32) | 80 (28) | 81 (35) | 29 (43) | 0.02 | 0.51 |
| Class IV, n (%) | 1 (0) | 9 (3) | 0 (0) | 1 (1) | 0.05 | 0.11 |
| Heart glycosides, n (%) | 32 (5) | 0 (0) | 17 (7) | 6 (9) | 0.15 | 0.25 |
| Selective sinus node inhibitors, n (%) | 16 (3) | 4 (1) | 9 (3) | 3 (4) | 0.00 | 0.41 |
| ACE-I/ARB/ARNI, n (%) | 450 (77) | 202 (71) | 191 (81) | 57 (84) | 0.00 | 0.09 |
| MRA, n (%) | 204 (35) | 52 (18) | 117 (50) | 35 (51) | 0.00 | 0.00 |
| Loop diuretics, n (%) | 287 (49) | 86 (30) | 153 (65) | 48 (71) | 0.62 | 0.00 |
| Thiazides, n (%) | 54 (9) | 27 (10) | 23 (10) | 4 (6) | 0.01 | 0.50 |
| SGLT-2 inhibitor, n (%) | 11 (2) | 1 (0) | 7 (3) | 3 (4) | 0.00 | 0.12 |
| Anticoagulation/antiplatelet therapy | ||||||
| Salicylates, n (%) | 139 (24) | 74 (26) | 54 (23) | 11 (16) | 0.11 | 0.17 |
| P2Y12 blockers, n (%) | 36 (6) | 19 (7) | 10 (4) | 7 (10) | 0.72 | 0.17 |
| Vitamin K antagonist, n (%) | 301 (51) | 115 (40) | 151 (64) | 35 (51) | 0.00 | 0.90 |
| DOAC, n (%) | 52 (9) | 22 (8) | 13 (6) | 17 (25) | 0.00 | 0.00 |
| Polypharmacy *, n (%) | 461 (78) | 194 (68) | 206 (88) | 61 (90) | 0.00 | 0.01 |
| Mortality | 1-Year Mortality (n = 588) | ICD Exchange (n = 268) | CRT-D Exchange (n = 234) | CRT-D Upgrade (n = 68) | p-Value ICD vs. CRT | p-Value CRT Exchange vs. Upgrade |
|---|---|---|---|---|---|---|
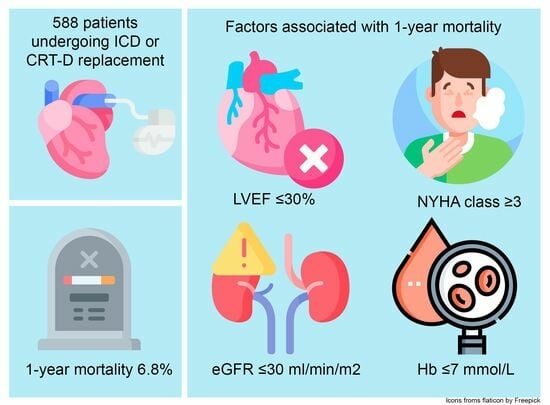
| All-cause mortality %, (n) | 6.8% (40) | 2.8% (8) | 10.7% (25) | 11.9% (7) | 0.002 | 0.68 |
| Cardiac mortality %, (n) | 53% (21) | 25% (2) | 68% (17) | 86% (6) | 0.35 | 0.83 |
| Heart failure | 100% (21) | |||||
| Arrythmia-related death | 0 | |||||
| Non-cardiac mortality | 47% (19) | 75% (6) | 32% (8) | 14.% (1) | ||
| Cancer | 32% (6) | |||||
| Renal failure | 16% (3) | |||||
| Pulmonary disease | 21% (4) | |||||
| Infectious disease | 21% (4) | |||||
| Neurological disease | 11% (2) |
| HR | 95% CI | p-Value | |
|---|---|---|---|
| Gender | 0.589 | 0.25–1.40 | 0.230 |
| Age ≥ 75 years | 2.15 | 1.15–4.02 | 0.017 |
| BMI in kg/m2 | 1.00 | 0.95–1.05 | 0.946 |
| NYHA class ≥ 3 | 5.92 | 2.81–9.92 | 0.000 |
| CIED | |||
| Primary prevention | 0.78 | 0.41–1.50 | 0.455 |
| Previous device infection | 2.76 | 1.08–7.04 | 0.034 |
| Number of pulse generator exchanges | 1.05 | 0.697–1.589 | 0.808 |
| Type of exchange | |||
| ICD | 0.002 | ||
| CRT-D exchange | 3.94 | 1.78–8.74 | 0.001 |
| CRT-D upgrade | 3.96 | 1.44–10.93 | 0.008 |
| Cardiac condition | |||
| Structural heart disease | 1.68 | 0.41–6.95 | 0.476 |
| Electrical heart disease | 0.31 | 0.19–4.87 | 0.401 |
| Cardiac history | |||
| Atrial fibrillation | 2.22 | 1.18–4.17 | 0.014 |
| Previous PCI | 1.06 | 0.55–2.06 | 0.854 |
| Previous CABG | 1.29 | 0.63–2.64 | 0.484 |
| Previous valve surgery | 1.55 | 0.79–3.05 | 0.203 |
| Echocardiographic findings | |||
| LVEF ≤ 30% | 3.76 | 1.99–7.12 | 0.000 |
| RVF | 3.20 | 0.71–14.02 | 0.122 |
| sPAP | 1.03 | 1.01–1.09 | 0.007 |
| Comorbidities and risk factors | |||
| Diabetes mellitus | 2.01 | 1.07–3.98 | 0.039 |
| COPD | 1.10 | 0.51–2.38 | 0.815 |
| Hypercholesteremia | 1.37 | 0.72–2.60 | 0.332 |
| CVA/TIA | 1.69 | 0.75–3.81 | 0.211 |
| Gastro-intestinal disease | 1.41 | 0.59–3.36 | 0.436 |
| History of malignancy | 0.92 | 0.36–2.35 | 0.864 |
| Laboratory findings | |||
| eGFR ≤ 30 mL/min/1.73 m2 | 6.91 | 3.57–13.40 | 0.000 |
| Potassium in mmol/L | 3.91 | 0.57–2.16 | 0.772 |
| Sodium in mmol/L | 0.99 | 0.97–1.01 | 0.360 |
| Haemoglobin ≤ 7 mmol/L | 5.72 | 2.95–11.09 | 0.000 |
| HR | 95% CI | p Value | |
|---|---|---|---|
| LVEF ≤ 30% | 2.41 | 1.20–4.83 | 0.013 |
| NYHA class ≥ 3 | 2.85 | 1.41–5.74 | 0.003 |
| eGFR ≤ 30 mL/min/1.73 m2 | 3.92 | 1.89–8.11 | <0.001 |
| Hb < 7 mmol/L | 3.09 | 1.50–6.37 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feijen, M.; Egorova, A.D.; Kuijken, T.; Bootsma, M.; Schalij, M.J.; van Erven, L. One-Year Mortality in Patients Undergoing an Implantable Cardioverter Defibrillator or Cardiac Resynchronization Therapy Pulse Generator Replacement: Identifying Patients at Risk. J. Clin. Med. 2023, 12, 5654. https://doi.org/10.3390/jcm12175654
Feijen M, Egorova AD, Kuijken T, Bootsma M, Schalij MJ, van Erven L. One-Year Mortality in Patients Undergoing an Implantable Cardioverter Defibrillator or Cardiac Resynchronization Therapy Pulse Generator Replacement: Identifying Patients at Risk. Journal of Clinical Medicine. 2023; 12(17):5654. https://doi.org/10.3390/jcm12175654
Chicago/Turabian StyleFeijen, Michelle, Anastasia D. Egorova, Teresa Kuijken, Marianne Bootsma, Martin J. Schalij, and Lieselot van Erven. 2023. "One-Year Mortality in Patients Undergoing an Implantable Cardioverter Defibrillator or Cardiac Resynchronization Therapy Pulse Generator Replacement: Identifying Patients at Risk" Journal of Clinical Medicine 12, no. 17: 5654. https://doi.org/10.3390/jcm12175654
APA StyleFeijen, M., Egorova, A. D., Kuijken, T., Bootsma, M., Schalij, M. J., & van Erven, L. (2023). One-Year Mortality in Patients Undergoing an Implantable Cardioverter Defibrillator or Cardiac Resynchronization Therapy Pulse Generator Replacement: Identifying Patients at Risk. Journal of Clinical Medicine, 12(17), 5654. https://doi.org/10.3390/jcm12175654