Repetitive Transcranial Magnetic Stimulation (rTMS) in Mild Cognitive Impairment: Effects on Cognitive Functions—A Systematic Review
Abstract
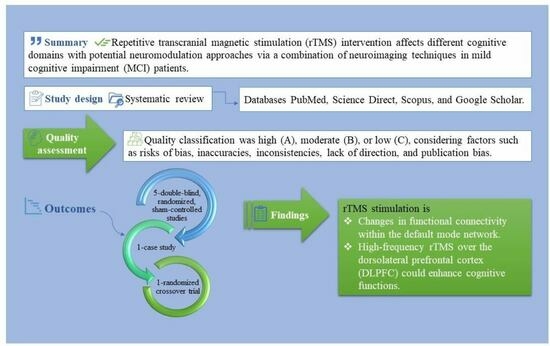
:1. Introduction
2. Methods
2.1. Study Focus
2.2. Inclusion and Exclusion Criteria
2.3. Search Strategy
2.4. Quality Assessment, Study Screening, and Risk of Bias
2.5. Data Items
3. Results
3.1. Study Selection
3.2. Studies’ Characteristics and Patient Demographics
3.3. Cognitive and Neuroimaging Findings after rTMS Stimulation
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, N.D. State of the science on mild cognitive impairment (MCI). CNS Spectr. 2019, 24, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.D.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S.; et al. Practice guideline update summary: Mild cognitive impairment: Report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Hugo, J.; Ganguli, M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef] [PubMed]
- Jongsiriyanyong, S.; Limpawattana, P. Mild Cognitive Impairment in Clinical Practice: A Review Article. Am. J. Alzheimer’s Dis. Other Dement. 2018, 33, 500–507. [Google Scholar] [CrossRef]
- Drumond Marra, H.L.; Myczkowski, M.L.; Maia Memória, C.; Arnaut, D.; Leite Ribeiro, P.; Sardinha Mansur, C.G.; Lancelote Alberto, R.; Boura Bellini, B.; Alves Fernandes da Silva, A.; Tortella, G.; et al. Transcranial Magnetic Stimulation to Address Mild Cognitive Impairment in the Elderly: A Randomized Controlled Study. Behav. Neurol. 2015, 2015, 287843. [Google Scholar] [CrossRef]
- Pang, Y.; Shi, M. Repetitive Transcranial Magnetic Stimulation Improves Mild Cognitive Impairment Associated with Alzheimer’s Disease in Mice by Modulating the miR-567/NEUROD2/PSD95 Axis. Neuropsychiatr. Dis. Treat. 2021, 17, 2151–2161. [Google Scholar] [CrossRef]
- Breton, A.; Casey, D.; Arnaoutoglou, N.A. Cognitive tests for the detection of mild cognitive impairment (MCI), the prodromal stage of dementia: Meta-analysis of diagnostic accuracy studies. Int. J. Geriatr. Psychiatry 2019, 34, 233–242. [Google Scholar] [CrossRef]
- Luis, C.A.; Loewenstein, D.A.; Acevedo, A.; Barker, W.W.; Duara, R. Mild cognitive impairment: Directions for future research. Neurology 2003, 61, 438–444. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Vega, J.N.; Newhouse, P.A. Mild cognitive impairment: Diagnosis, longitudinal course, and emerging treatments. Curr. Psychiatry Rep. 2014, 16, 490. [Google Scholar] [CrossRef] [PubMed]
- Sachs-Ericsson, N.; Blazer, D.G. The new DSM-5 diagnosis of mild neurocognitive disorder and its relation to research in mild cognitive impairment. Aging Ment. Health 2015, 19, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Lopez, O.L. Mild cognitive impairment. Continuum 2013, 19, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef]
- Ozdemir, R.A.; Tadayon, E.; Boucher, P.; Momi, D.; Karakhanyan, K.A.; Fox, M.D.; Halko, M.A.; Pascual-Leone, A.; Shafi, M.M.; Santarnecchi, E. Individualized perturbation of the human connectome reveals reproducible biomarkers of network dynamics relevant to cognition. Proc. Natl. Acad. Sci. USA 2020, 117, 8115–8125. [Google Scholar] [CrossRef]
- Pievani, M.; Mega, A.; Quattrini, G.; Guidali, G.; Ferrari, C.; Cattaneo, A.; D’Aprile, I.; Mascaro, L.; Gasparotti, R.; Corbo, D.; et al. Targeting Default Mode Network Dysfunction in Persons at Risk of Alzheimer’s Disease with Transcranial Magnetic Stimulation (NEST4AD): Rationale and Study Design. J. Alzheimers Dis. 2021, 83, 1877–1889. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Bella, R.; Benussi, A.; Bologna, M.; Borroni, B.; Capone, F.; Chen, K.S.; Chen, R.; Chistyakov, A.V.; Classen, J.; et al. Diagnostic contribution and therapeutic perspectives of transcranial magnetic stimulation in dementia. Clin. Neurophysiol. 2021, 132, 2568–2607. [Google Scholar] [CrossRef]
- Cheng, C.P.W.; Wong, C.S.M.; Lee, K.K.; Chan, A.P.K.; Yeung, J.W.F.; Chan, W.C. Effects of repetitive transcranial magnetic stimulation on improvement of cognition in elderly patients with cognitive impairment: A systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2018, 33, e1–e13. [Google Scholar] [CrossRef]
- Sliwinska, M.W.; Vitello, S.; Devlin, J.T. Transcranial magnetic stimulation for investigating causal brain-behavioral relationships and their time course. J. Vis. Exp. 2014, 89, 51735. [Google Scholar] [CrossRef]
- Somaa, F.A.; de Graaf, T.A.; Sack, A.T. Transcranial Magnetic Stimulation in the Treatment of Neurological Diseases. Front. Neurol. 2022, 13, 793253. [Google Scholar] [CrossRef]
- Bagattini, C.; Zanni, M.; Barocco, F.; Caffarra, P.; Brignani, D.; Miniussi, C.; Defanti, C.A. Enhancing cognitive training effects in Alzheimer’s disease: rTMS as an add-on treatment. Brain Stimul. 2020, 13, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Müller-Dahlhaus, F.; Vlachos, A. Unraveling the cellular and molecular mechanisms of repetitive magnetic stimulation. Front. Mol. Neurosci. 2013, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef]
- Chervyakov, A.V.; Chernyavsky, A.Y.; Sinitsyn, D.O.; Piradov, M.A. Possible Mechanisms Underlying the Therapeutic Effects of Transcranial Magnetic Stimulation. Front. Hum. Neurosci. 2015, 9, 303. [Google Scholar] [CrossRef]
- Li, C.T.; Huang, Y.Z.; Bai, Y.M.; Tsai, S.J.; Su, T.P.; Cheng, C.M. The critical role of glutamatergic and GABAergic neurotransmission in the central mechanisms of theta-burst stimulation. Hum. Brain Mapp. 2019, 40, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P. Transcranial magnetic stimulation. Handb. Clin. Neurol. 2019, 160, 559–580. [Google Scholar] [CrossRef]
- Machado, S.; Arias-Carrión, O.; Paes, F.; Vieira, R.T.; Caixeta, L.; Novaes, F.; Marinho, T.; Almada, L.F.; Silva, A.C.; Nardi, A.E. Repetitive transcranial magnetic stimulation for clinical applications in neurological and psychiatric disorders: An overview. Eurasian J. Med. 2013, 45, 191–206. [Google Scholar] [CrossRef]
- Maeda, F.; Keenan, J.P.; Tormos, J.M.; Topka, H.; Pascual-Leone, A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp. Brain Res. 2000, 133, 425–430. [Google Scholar] [CrossRef]
- Cárdenas-Morales, L.; Nowak, D.A.; Kammer, T.; Wolf, R.C.; Schönfeldt-Lecuona, C. Mechanisms and applications of theta-burst rTMS on the human motor cortex. Brain Topogr. 2010, 22, 294–306. [Google Scholar] [CrossRef]
- Tik, M.; Hoffmann, A.; Sladky, R.; Tomova, L.; Hummer, A.; Navarro de Lara, L.; Bukowski, H.; Pripfl, J.; Biswal, B.; Lamm, C.; et al. Towards understanding rTMS mechanism of action: Stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage 2017, 162, 289–296. [Google Scholar] [CrossRef]
- Pulopulos, M.M.; Allaert, J.; Vanderhasselt, M.A.; Sanchez-Lopez, A.; De Witte, S.; Baeken, C.; De Raedt, R. Effects of HF-rTMS over the left and right DLPFC on proactive and reactive cognitive control. Soc. Cogn. Affect. Neurosci. 2022, 17, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Pell, G.S.; Roth, Y.; Zangen, A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: Influence of timing and geometrical parameters and underlying mechanisms. Prog. Neurobiol. 2011, 93, 59–98. [Google Scholar] [CrossRef] [PubMed]
- Gevins, A.; Leong, H.; Smith, M.E.; Le, J.; Du, R. Mapping cognitive brain function with modern high-resolution electroencephalography. Trends Neurosci. 1995, 18, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.J.; Gupta, D.; Brunner, P.; Gunduz, A.; Adamo, M.A.; Ritaccio, A.; Schalk, G. Recording human electrocorticographic (ECoG) signals for neuroscientific research and real-time functional cortical mapping. J. Vis. Exp. 2012, 64, 3993. [Google Scholar] [CrossRef]
- Nardone, R.; Tezzon, F.; Höller, Y.; Golaszewski, S.; Trinka, E.; Brigo, F. Transcranial magnetic stimulation (TMS)/repetitive TMS in mild cognitive impairment and Alzheimer’s disease. Acta Neurol. Scand. 2014, 129, 351–366. [Google Scholar] [CrossRef]
- Cao, K.X.; Ma, M.L.; Wang, C.Z.; Iqbal, J.; Si, J.J.; Xue, Y.X.; Yang, J.L. TMS-EEG: An emerging tool to study the neurophysiologic biomarkers of psychiatric disorders. Neuropharmacology 2021, 197, 108574. [Google Scholar] [CrossRef]
- Babiloni, C.; Del Percio, C.; Pascarelli, M.T.; Lizio, R.; Noce, G.; Lopez, S.; Rizzo, M.; Ferri, R.; Soricelli, A.; Nobili, F.; et al. Abnormalities of functional cortical source connectivity of resting-state electroencephalographic alpha rhythms are similar in patients with mild cognitive impairment due to Alzheimer’s and Lewy body diseases. Neurobiol. Aging 2019, 77, 112–127. [Google Scholar] [CrossRef]
- Boutros, N.N.; Berman, R.M.; Hoffman, R.; Miano, A.P.; Campbell, D.; Ilmoniemi, R. Electroencephalogram and repetitive transcranial magnetic stimulation. Depress. Anxiety 2000, 12, 166–169. [Google Scholar] [CrossRef]
- Ilmoniemi, R.J.; Kicić, D. Methodology for combined TMS and EEG. Brain Topogr. 2010, 22, 233–248. [Google Scholar] [CrossRef]
- Song, P.; Lin, H.; Li, S.; Wang, L.; Liu, J.; Li, N.; Wang, Y. Repetitive transcranial magnetic stimulation (rTMS) modulates time-varying electroencephalography (EEG) network in primary insomnia patients: A TMS-EEG study. Sleep Med. 2019, 56, 157–163. [Google Scholar] [CrossRef]
- Li, F.; Peng, W.; Jiang, Y.; Song, L.; Liao, Y.; Yi, C.; Zhang, L.; Si, Y.; Zhang, T.; Wang, F.; et al. The Dynamic Brain Networks of Motor Imagery: Time-Varying Causality Analysis of Scalp EEG. Int. J. Neural Syst. 2019, 29, 1850016. [Google Scholar] [CrossRef] [PubMed]
- Thut, G.; Miniussi, C. New insights into rhythmic brain activity from TMS-EEG studies. Trends Cogn. Sci. 2009, 13, 182–189. [Google Scholar] [CrossRef]
- Thut, G.; Pascual-Leone, A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 2010, 22, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Pievani, M.; Filippini, N.; van den Heuvel, M.P.; Cappa, S.F.; Frisoni, G.B. Brain connectivity in neurodegenerative diseases--from phenotype to proteinopathy. Nat. Rev. Neurol. 2014, 10, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Hamidi, M.; Postle, B.R. Using EEG to explore how rTMS produces its effects on behavior. Brain Topogr. 2010, 22, 281–293. [Google Scholar] [CrossRef]
- Abellaneda-Pérez, K.; Vaqué-Alcázar, L.; Solé-Padullés, C.; Bartrés-Faz, D. Combining non-invasive brain stimulation with functional magnetic resonance imaging to investigate the neural substrates of cognitive aging. J. Neurosci. Res. 2022, 100, 1159–1170. [Google Scholar] [CrossRef]
- Iacoboni, M. The role of premotor cortex in speech perception: Evidence from fMRI and rTMS. J. Physiol. Paris 2008, 102, 31–34. [Google Scholar] [CrossRef]
- Binney, R.J.; Ralph, M.A. Using a combination of fMRI and anterior temporal lobe rTMS to measure intrinsic and induced activation changes across the semantic cognition network. Neuropsychologia 2015, 76, 170–181. [Google Scholar] [CrossRef]
- Fox, M.D.; Buckner, R.L.; Liu, H.; Chakravarty, M.M.; Lozano, A.M.; Pascual-Leone, A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc. Natl. Acad. Sci. USA 2014, 111, E4367–E4375. [Google Scholar] [CrossRef]
- Zheng, A.; Yu, R.; Du, W.; Liu, H.; Zhang, Z.; Xu, Z.; Xiang, Y.; Du, L. Two-week rTMS-induced neuroimaging changes measured with fMRI in depression. J. Affect. Disord. 2020, 270, 15–21. [Google Scholar] [CrossRef]
- Ruff, C.C.; Driver, J.; Bestmann, S. Combining TMS and fMRI: From ‘virtual lesions’ to functional-network accounts of cognition. Cortex 2009, 45, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Sack, A.T.; Hubl, D.; Prvulovic, D.; Formisano, E.; Jandl, M.; Zanella, F.E.; Maurer, K.; Goebel, R.; Dierks, T.; Linden, D.E. The experimental combination of rTMS and fMRI reveals the functional relevance of parietal cortex for visuospatial functions. Brain Res. Cogn. Brain Res. 2002, 13, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Bagattini, C.; Brignani, D.; Bonnì, S.; Quattrini, G.; Gasparotti, R.; Pievani, M. Functional Imaging to Guide Network-Based TMS Treatments: Toward a Tailored Medicine Approach in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 687493. [Google Scholar] [CrossRef]
- Uzair, M.; Abualait, T.; Arshad, M.; Yoo, W.K.; Mir, A.; Bunyan, R.F.; Bashir, S. Transcranial magnetic stimulation in animal models of neurodegeneration. Neural Regen. Res. 2022, 17, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, P.; Lu, S.; Li, K.; Zhong, N. The role of the DLPFC in inductive reasoning of MCI patients and normal agings: An fMRI study. Sci. China C Life Sci. 2009, 52, 789–795. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Yuan, L.Q.; Zeng, Q.; Wang, D.; Wen, X.Y.; Shi, Y.; Zhu, F.; Chen, S.J.; Huang, G.Z. Neuroimaging mechanisms of high-frequency repetitive transcranial magnetic stimulation for treatment of amnestic mild cognitive impairment: A double-blind randomized sham-controlled trial. Neural Regen. Res. 2021, 16, 707–713. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Roberts, R.; Knopman, D.S. Classification and epidemiology of MCI. Clin. Geriatr. Med. 2013, 29, 753–772. [Google Scholar] [CrossRef]
- Chu, C.S.; Li, C.T.; Brunoni, A.R.; Yang, F.C.; Tseng, P.T.; Tu, Y.K.; Stubbs, B.; Carvalho, A.F.; Thompson, T.; Rajji, T.K.; et al. Cognitive effects and acceptability of non-invasive brain stimulation on Alzheimer’s disease and mild cognitive impairment: A component network meta-analysis. J. Neurol. Neurosurg. Psychiatry 2021, 92, 195–203. [Google Scholar] [CrossRef]
- Jiang, L.; Cui, H.; Zhang, C.; Cao, X.; Gu, N.; Zhu, Y.; Wang, J.; Yang, Z.; Li, C. Repetitive Transcranial Magnetic Stimulation for Improving Cognitive Function in Patients with Mild Cognitive Impairment: A Systematic Review. Front. Aging Neurosci. 2021, 12, 593000. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.H.; Ton That, V.; Sundman, M. A systematic review and meta-analysis of rTMS effects on cognitive enhancement in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2020, 86, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Padala, P.R.; Padala, K.P.; Lensing, S.Y.; Jackson, A.N.; Hunter, C.R.; Parkes, C.M.; Dennis, R.A.; Bopp, M.M.; Caceda, R.; Mennemeier, M.S.; et al. Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: A double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res. 2018, 261, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Bouaziz, N.; Braha-Zeitoun, S.; Isaac, C.; Schenin-King Andrianisaina, P.; Januel, D. The Positive Effect of Long-Term Repetitive Transcranial Magnetic Stimulation Therapy for Mild Cognitive Impairment: Three Case Studies. OBM Geriatr. 2018, 2, 5. [Google Scholar] [CrossRef]
- Cui, H.; Ren, R.; Lin, G.; Zou, Y.; Jiang, L.; Wei, Z.; Li, C.; Wang, G. Repetitive Transcranial Magnetic Stimulation Induced Hypoconnectivity within the Default Mode Network Yields Cognitive Improvements in Amnestic Mild Cognitive Impairment: A Randomized Controlled Study. J. Alzheimers Dis. 2019, 69, 1137–1151. [Google Scholar] [CrossRef]
- Taylor, J.L.; Hambro, B.C.; Strossman, N.D.; Bhatt, P.; Hernandez, B.; Ashford, J.W.; Cheng, J.J.; Iv, M.; Adamson, M.M.; Lazzeroni, L.C.; et al. The effects of repetitive transcranial magnetic stimulation in older adults with mild cognitive impairment: A protocol for a randomized, controlled three-arm trial. BMC Neurol. 2019, 19, 326. [Google Scholar] [CrossRef]
- Roque Roque, G.Y.; Reyes-López, J.V.; Ricardo Garcell, J.; Ricardo Garcell, J.; López Hidalgo, M.; Aguilar Fabré, L.; Trejo Cruz, G.; Cañizares Gómez, S.; Calderón Moctezuma, A.R.; Ortega Cruz, F.; et al. Effect of transcranial magnetic stimulation as an enhancer of cognitive stimulation sessions on mild cognitive impairment: Preliminary results. Psychiatry Res. 2021, 304, 114151. [Google Scholar] [CrossRef]
- Esposito, S.; Trojsi, F.; Cirillo, G.; de Stefano, M.; Di Nardo, F.; Siciliano, M.; Caiazzo, G.; Ippolito, D.; Ricciardi, D.; Buonanno, D.; et al. Repetitive Transcranial Magnetic Stimulation (rTMS) of Dorsolateral Prefrontal Cortex May Influence Semantic Fluency and Functional Connectivity in Fronto-Parietal Network in Mild Cognitive Impairment (MCI). Biomedicines 2022, 10, 994. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Yuan, Y.; Fan, Y.; Liu, F.; Zhu, J.; Xu, Q.; Chen, L.; Guo, M.; Ji, Z.; et al. Effects of high frequency rTMS of contralesional dorsal premotor cortex in severe subcortical chronic stroke: Protocol of a randomized controlled trial with multimodal neuroimaging assessments. BMC Neurol. 2022, 22, 125. [Google Scholar] [CrossRef]
- Cha, B.; Kim, J.; Kim, J.M.; Choi, J.W.; Choi, J.; Kim, K.; Cha, J.; Kim, M. Therapeutic Effect of Repetitive Transcranial Magnetic Stimulation for Post-stroke Vascular Cognitive Impairment: A Prospective Pilot Study. Front. Neurol. 2022, 13, 813597. [Google Scholar] [CrossRef]
- Pennisi, M.; Lanza, G.; Cantone, M.; Ricceri, R.; Spampinato, C.; Pennisi, G.; Di Lazzaro, V.; Bella, R. Correlation between Motor Cortex Excitability Changes and Cognitive Impairment in Vascular Depression: Pathophysiological Insights from a Longitudinal TMS Study. Neural Plast. 2016, 2016, 8154969. [Google Scholar] [CrossRef] [PubMed]
- Bella, R.; Ferri, R.; Pennisi, M.; Cantone, M.; Lanza, G.; Malaguarnera, G.; Spampinato, C.; Giordano, D.; Alagona, G.; Pennisi, G. Enhanced motor cortex facilitation in patients with vascular cognitive impairment-no dementia. Neurosci. Lett. 2011, 503, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, X.; Lu, X.; Yang, Z.; Meng, Y.; Qie, H.; Dai, C.; Yu, W.; Han, J.; Ding, N.; et al. Beneficial effects of repetitive transcranial magnetic stimulation on cognitive function and self-care ability in patients with non-dementia vascular cognitive impairment. Int. J. Clin. Exp. Med. 2020, 13, 3197–3204. [Google Scholar]
- Oathes, D.J.; Balderston, N.L.; Kording, K.P.; DeLuisi, J.A.; Perez, G.M.; Medaglia, J.D.; Fan, Y.; Duprat, R.J.; Satterthwaite, T.D.; Sheline, Y.I.; et al. Combining transcranial magnetic stimulation with functional magnetic resonance imaging for probing and modulating neural circuits relevant to affective disorders. Wiley Interdiscip. Rev. Cogn. Sci. 2021, 12, e1553. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Ma, J.; Li, J.; Zhang, Z.; Wang, M. Repetitive Transcranial Magnetic Stimulation (rTMS) Modulates Lipid Metabolism in Aging Adults. Front. Aging Neurosci. 2017, 9, 334. [Google Scholar] [CrossRef]
- Zhu, S.; Wei, X.; Yang, X.; Huang, Z.; Chang, Z.; Xie, F.; Yang, Q.; Ding, C.; Xiang, W.; Yang, H.; et al. Plasma Lipoprotein-associated Phospholipase A2 and Superoxide Dismutase are Independent Predicators of Cognitive Impairment in Cerebral Small Vessel Disease Patients: Diagnosis and Assessment. Aging Dis. 2019, 10, 834–846. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, W.J.; Shan, P.Y.; Lu, M.; Wang, T.; Li, R.H.; Zhang, N.; Ma, L. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer’s disease: A systematic review and meta-analysis. J. Neurol. Sci. 2019, 398, 184–191. [Google Scholar] [CrossRef]
- Lee, J.; Choi, B.H.; Oh, E.; Sohn, E.H.; Lee, A.Y. Treatment of Alzheimer’s Disease with Repetitive Transcranial Magnetic Stimulation Combined with Cognitive Training: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Neurol. 2016, 12, 57–64. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, H.; Pei, Z.; Lian, C.; Su, X.; Lan, X.; Chen, C.; Lei, Y.; Li, B.; Guo, Y. Dual-targeted repetitive transcranial magnetic stimulation modulates brain functional network connectivity to improve cognition in mild cognitive impairment patients. Front. Physiol. 2022, 13, 1066290. [Google Scholar] [CrossRef]
- Beynel, L.; Appelbaum, L.G.; Luber, B.; Crowell, C.A.; Hilbig, S.A.; Lim, W.; Nguyen, D.; Chrapliwy, N.A.; Davis, S.W.; Cabeza, R.; et al. Effects of online repetitive transcranial magnetic stimulation (rTMS) on cognitive processing: A meta-analysis and recommendations for future studies. Neurosci. Biobehav. Rev. 2019, 107, 47–58. [Google Scholar] [CrossRef]
- Sacco, L.; Ceroni, M.; Pacifico, D.; Zerboni, G.; Rossi, S.; Galati, S.; Caverzasio, S.; Kaelin-Lang, A.; Riccitelli, G.C. Transcranial Magnetic Stimulation Improves Executive Functioning through Modulation of Social Cognitive Networks in Patients with Mild Cognitive Impairment: Preliminary Results. Diagnostics 2023, 13, 415. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Pepe, R.; Siciliano, M.; Ippolito, D.; Ricciardi, D.; de Stefano, M.; Buonanno, D.; Atripaldi, D.; Abbadessa, S.; Perfetto, B.; et al. Long-Term Neuromodulatory Effects of Repetitive Transcranial Magnetic Stimulation (rTMS) on Plasmatic Matrix Metalloproteinases (MMPs) Levels and Visuospatial Abilities in Mild Cognitive Impairment (MCI). Int. J. Mol. Sci. 2023, 24, 3231. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Y.; Nie, L.; Zhang, W.; Ke, Z.; Ku, Y. Cognitive Enhancement of Repetitive Transcranial Magnetic Stimulation in Patients with Mild Cognitive Impairment and Early Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Cell Dev. Biol. 2021, 9, 734046. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, G.; Yu, C.; Lian, G.; Zheng, H.; Wu, S.; Yuan, T.F.; Zhou, D. Cortical plasticity is correlated with cognitive improvement in Alzheimer’s disease patients after rTMS treatment. Brain Stimul. 2021, 14, 503–510. [Google Scholar] [CrossRef] [PubMed]

| Study, Year | Country | Disease | N | M/F | Age | Type of Study | |||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Sham | Treatment | Sham | Treatment | Sham | ||||
| Padala et al. [64], (2018) | United States | aMCI, naMCI | 4 | 5 | 4/0 | 4/1 | 68.0 ± 10.0 | 64.0 ± 9.0 | Double-blind, Randomized, Sham-controlled trial |
| Durand et al. [65], (2018) | France | aMCI, naMCI | 3 | N/A | 1/2 | N/A | 69 ± 6.65 | N/A | Three case studies |
| Cui et al. [66], (2019) | China | aMCI | 21 | N/A | N/A | N/A | 50–85 | 50–85 | Double-blind, Randomized, Sham-controlled trial |
| Taylor et al. [67], (2019) | United States | aMCI | 66 | 33 | N/A | N/A | 55–90 | 55–90 | Double-blind, Randomized, Sham-controlled, three-arm trial |
| Roque Roque et al. [68], (2021) | México | aMCI, naMCI | 11 | 11 | 5/7 | 3/9 | 66.1 ± 5.5 | 67.2 ± 4.8 | Randomized crossover trial |
| Yuan et al. [58], (2021) | China | aMCI | 12 | 12 | 6/6 | 5/7 | 65.08 ± 4.89 | 64.67 ± 4.77 | Double-blind, randomized, sham-controlled trial |
| Esposito et al. [69], (2022) | Italy | aMCI, naMCI | 27 | 13 | 14/13 | 5/8 | 67.85 ± 9.28 | 66.77 ± 9.08 | Double-blind, randomized, sham-controlled trial |
| Study, Year | Group | Intervention | Stimulation | Cognitive/Neuroimaging Findings | ORs, 95%Cis | Main Findings |
|---|---|---|---|---|---|---|
| Padala et al. [64], (2018) | Active r-TMS, sham-controlled | Non-navigated rTMS: 3000 pulses at 10 Hz, 4-s train duration, and 26-s inter-train interval, per session five times a week; % motor threshold: 120% | Left DLPFC | 1- Apathy (AES-C) | p < 0.001 | Significantly greater improvement in 3MS, MMSE, TMT-A, and CGI-I with rTMS compared to the sham treatment. |
| 2- Global cognition (3MS; global screen for cognition expanded from the MMSE) | p < 0.001 | |||||
| 3- Executive function (TMT-A & TMT-B) | p < 0.05 | |||||
| 4- Functional status (IADL) | p > 0.05 | |||||
| 5- Patient’s global functioning (CGI-S, CGI I) | p > 0.05; p < 0.001 | |||||
| 6- Caregiver burden (ZBS) | p > 0.05 | |||||
| Durand et al. [65], (2018) | Active rTMS | The 3 patients received non-navigated rTMS (i.e., 10 Hz, 1 Hz, and 50 Hz-burst) sessions from 1 to 4 times a week; % motor threshold: 110%, 80% | Left/right DLPFC | 1- Global cognition (MoCA) | N/A | The cognitive and clinical benefits of long-term rTMS treatment in MCI patients, without side effects, have been highlighted. This cognitive improvement is regardless of any anti-depressive effects. |
| 2- CGI-I | N/A | |||||
| 3- Depression (HDRS) | N/A | |||||
| Cui et al. [66], (2019) | Active rTMS, sham-controlled | Non-navigated rTMS: 30 trains of 5 s stimuli delivered at 10 Hz; 10-session daily treatment for about 2 weeks; % motor threshold: 90% | Right DLPFC | 1- Global cognition (MMSE, ACE-III) | p < 0.001 | rTMS-induced hypoconnectivity within DMN is associated with clinical cognitive improvements in patients with aMCI. |
| 2- Memory (Auditory Verbal Learning Test, AVLT, TMT-A & TMT-B) | p < 0.001 | |||||
| 3- Geriatric Depression Scale (GDS) | p > 0.05 | |||||
| 4- Functional connectivity (resting- state functional MRI) | p < 0.001 | |||||
| Taylor et al. [67], (2019) | Active rTMS, sham-controlled | Navigated rTMS:10Hz delivers, 4000 pulses per session and up to 8000 pulses per day, with a total of 80,000 pulses over 2- to 4-week period; % motor threshold: 120% | Bilateral DLPFC Bilateral Lateral parietal cortex (LPC) Sham control | 1- Memory (California Verbal Learning Test-II, CVLT-II) | p < 0.05 | Positive effects of rTMS on cognitive and neuroimaging outcomes (i.e., global cognitive function, mood, and neuroimaging biomarkers). |
| 2- Global cognitive function (MoCA) | p < 0.05 | |||||
| 3- Visuospatial episodic memory (BVMT-R) | p < 0.05 | |||||
| 4- Language (BNT) | p < 0.05 | |||||
| 5- Visuoconstructional function (ROCF) | p < 0.05 | |||||
| 6- Speed of processing and executive control (TMT) | p < 0.05 | |||||
| 7- Geriatric Depression Scale (GDS) | p < 0.05 | |||||
| 8- Functional connectivity (resting state functional MRI) | p < 0.05 | |||||
| Roque Roque et al. [68], (2021) | Active rTMS, sham-controlled | Non-navigated rTMS: 1500 pulses (30 trains of 50 pulses, each with a 10-s intertrain interval), at 5 Hz, for 30 sessions; % motor threshold: 100% | Left DLPFC | 1- Global cognition (MoCA, MMSE) | p < 0.05 | Statistically significant in the intergroup analysis with MoCA and intragroup only for the Active group. |
| 2- Mental health assessment (Mini-International Neuropsychiatric Interview, GDS) | p < 0.05 | |||||
| 3- Neuropsychological assessment (NEUROPSI, ROCF, Stroop effect, and digit detection) | p < 0.05 | |||||
| 4- Electroencephalographic (EEG) examination | N/A | |||||
| Yuan et al. [58], (2021) | Active rTMS, sham-controlled | Non-navigated rTMS: frequency of 10 Hz, five times per week over a period of 4 consecutive weeks; % motor threshold: 80% | Left DLPFC | 1- Neuropsychological assessment (Clinical Dementia Rating Scale, Global Deterioration Scale, and MoCA) | p < 0.05 | High-frequency rTMS can effectively improve cognitive function in aMCI patients and alter spontaneous brain activity. |
| 2- RS-fMRI (pre-processing and ALFF analysis) | p < 0.05 | |||||
| Esposito et al. [69], (2022) | Active rTMS, sham-controlled | Non-navigated rTMS: frequency of 10 Hz, five times per week over a period of 4 consecutive weeks; % motor threshold: 80% | Bilateral DLPFC | 1- Global cognition (RBANS) | p < 0.001 | Significant long-term increase in FC in MCI patients in RS networks associated with executive functions. |
| 2- Beck Depression Inventory II | p > 0.05 | |||||
| 3- Beck Anxiety Inventory | p > 0.05 | |||||
| 4- AES | p ≤ 0.01 | |||||
| 5- Functional connectivity (resting state functional MRI) | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharbafshaaer, M.; Gigi, I.; Lavorgna, L.; Esposito, S.; Bonavita, S.; Tedeschi, G.; Esposito, F.; Trojsi, F. Repetitive Transcranial Magnetic Stimulation (rTMS) in Mild Cognitive Impairment: Effects on Cognitive Functions—A Systematic Review. J. Clin. Med. 2023, 12, 6190. https://doi.org/10.3390/jcm12196190
Sharbafshaaer M, Gigi I, Lavorgna L, Esposito S, Bonavita S, Tedeschi G, Esposito F, Trojsi F. Repetitive Transcranial Magnetic Stimulation (rTMS) in Mild Cognitive Impairment: Effects on Cognitive Functions—A Systematic Review. Journal of Clinical Medicine. 2023; 12(19):6190. https://doi.org/10.3390/jcm12196190
Chicago/Turabian StyleSharbafshaaer, Minoo, Ilaria Gigi, Luigi Lavorgna, Sabrina Esposito, Simona Bonavita, Gioacchino Tedeschi, Fabrizio Esposito, and Francesca Trojsi. 2023. "Repetitive Transcranial Magnetic Stimulation (rTMS) in Mild Cognitive Impairment: Effects on Cognitive Functions—A Systematic Review" Journal of Clinical Medicine 12, no. 19: 6190. https://doi.org/10.3390/jcm12196190
APA StyleSharbafshaaer, M., Gigi, I., Lavorgna, L., Esposito, S., Bonavita, S., Tedeschi, G., Esposito, F., & Trojsi, F. (2023). Repetitive Transcranial Magnetic Stimulation (rTMS) in Mild Cognitive Impairment: Effects on Cognitive Functions—A Systematic Review. Journal of Clinical Medicine, 12(19), 6190. https://doi.org/10.3390/jcm12196190