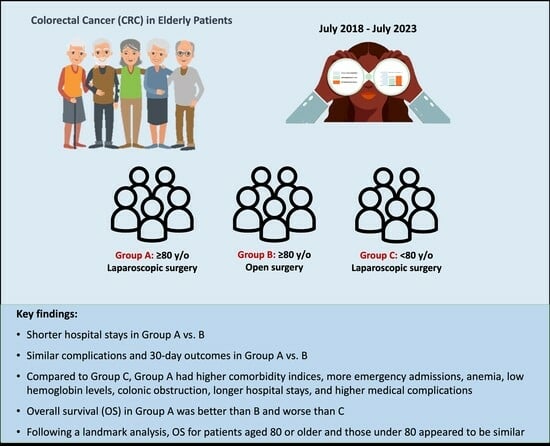
Outcomes of Laparoscopic Surgery in Very Elderly Patients with Colorectal Cancer: A Survival Analysis and Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
- Demographic information;
- Medical history and Charlson Comorbidity Index (CCI);
- Clinical symptoms at presentation;
- Pre-operative findings (e.g., colonoscopy, computed tomography, pelvic magnetic resonance imaging for rectal tumors);
- Perioperative data (type of resection, laparoscopy/open);
- Classification as an elective or emergent operation;
- Incidences of conversion to laparotomy;
- Post-operative medical and surgical complications, including those classified as Clavien–Dindo grade >2;
- Length of hospital stay;
- 30-day outcome, categorized as deceased, discharged, or still hospitalized;
- Sample histology and immunohistochemistry;
- Disease stage;
- Any adjuvant treatment;
- Signs and timing of cancer recurrence during follow-up;
- Causes and timings of patient deaths.
Statistical Analysis
3. Results
3.1. Patient Group Selection and Demographics
3.2. Comparison of Group A and Group B
3.3. Comparison of Group A and Group C
3.4. Risk Factor Analysis for Laparoscopic Groups (Group A and Group C)
3.5. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- I Numeri del Cancro in Italia. 2020. Available online: https://www.registri-tumori.it/cms/pubblicazioni/i-numeri-del-cancro-italia-2020 (accessed on 2 October 2021).
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.A. Colon cancer screening in the elderly: When do we stop. Trans. Am. Clin. Climatol. Assoc. 2010, 121, 94–103. [Google Scholar] [PubMed]
- Artiles-Armas, M.; Roque-Castellano, C.; Fariña-Castro, R.; Conde-Martel, A.; Acosta-Mérida, M.A.; Marchena-Gómez, J. Impact of frailty on 5-year survival in patients older than 70 years undergoing colorectal surgery for cancer. World J. Surg. Oncol. 2021, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Antonio, M.; García Valdecasas, J.C.; Delgado, S.; Castells, A.; Taurá, P.; Mpiqué, J.; Visa, J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: A randomised trial. Lancet 2002, 359, 2224–2229. [Google Scholar]
- Jacobs, M.; Verdeja, J.C.; Goldstein, H.S. Minimally invasive colon resection (laparoscopic colectomy). Surg. Laparosc. Endosc. 1991, 1, 144–150. [Google Scholar]
- Ishibe, A.; Ota, M.; Fujii, S.; Suwa, Y.; Suzuki, S.; Suwa, H.; Momiyama, M.; Watanabe, J.; Watanabe, K.; Taguri, M.; et al. Midterm follow-up of a randomized trial of open surgery versus laparoscopic surgery in elderly patients with colorectal cancer. Surg. Endosc. 2017, 31, 3890–3897. [Google Scholar] [CrossRef]
- Seishima, R.; Okabayashi, K.; Hasegawa, H.; Tsuruta, M.; Shigeta, K.; Matsui, S.; Yamada, T.; Kitagawa, Y. Is laparoscopic colorectal surgery benefcial for elderly patients? A systematic review and meta-analysis. J. Gastrointest. Surg. 2015, 19, 756–765. [Google Scholar] [CrossRef]
- Boller, A.M.; Nelson, H. Colon and rectal cancer: Laparoscopic or open? Clin. Cancer Res. 2007, 13, 6894–6896. [Google Scholar] [CrossRef]
- Veldkamp, R.; Gholghesai, M.; Bonjer, H.J.; Meijer, D.H.; Buunen, M.; Jeekel, J.; Anderberg, B.; Cuesta, M.A.; Cuschieri, A.; Fingerhut, A.; et al. Laparoscopic resection for colon cancer. Consensus of the European Association of Endoscopic Surgery (EAES). Surg. Endosc. 2004, 18, 1163–1185. [Google Scholar] [CrossRef]
- Guillou, P.J.; Quirke, P.; Thorpe, H.; Walker, J.; Jayne, D.G.; Smith, A.M.; Heath, R.M.; Brown, J.M.; MRC CLASICC Trial Group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): Multicentre, randomised controlled trial. Lancet 2005, 365, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Chern, Y.J.; Hung, H.Y.; You, J.F.; Hsu, Y.J.; Chiang, J.M.; Hsieh, P.S.; Tsai, W.S. Advantage of laparoscopy surgery for elderly colorectal cancer patients without compromising oncologic outcome. BMC Surg. 2020, 20, 294. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, V.; Conte, C.; D’Indinosante, M.; Federico, A.; Biscione, A.; Vizzielli, G.; Bottoni, C.; Carbone, M.V.; Legge, F.; Uccella, S.; et al. Robotic Surgery in Elderly and Very Elderly Gynecologic Cancer Patients. J. Minim. Invasive Gynecol. 2018, 25, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Ishihara, S.; Hata, K.; Murono, K.; Yasuda, K.; Otani, K.; Tanaka, T.; Kiyomatsu, T.; Kawai, K.; Nozawa, H.; et al. Short-term outcomes of open versus laparoscopic surgery in elderly patients with colorectal cancer. Surg. Endosc. 2016, 30, 5550–5557. [Google Scholar] [CrossRef]
- Honda, M.; Kumamaru, H.; Etoh, T.; Miyata, H.; Yamashita, Y.; Yoshida, K.; Kodera, Y.; Kakeji, Y.; Inomata, M.; Konno, H.; et al. Surgical risk and benefits of laparoscopic surgery for elderly patients with gastric cancer: A multicenter prospective cohort study. Gastric Cancer 2019, 22, 845–852. [Google Scholar] [CrossRef]
- Chen, K.; Pan, Y.; Maher, H.; Zhang, B.; Zheng, X.Y. Laparoscopic hepatectomy for elderly patients: Major findings based on a systematic review and meta-analysis. Medicine 2018, 97, e11703. [Google Scholar] [CrossRef]
- Ke, J.; Liu, Y.; Liu, F.; Ji, B. Application of Laparoscopic Pancreatoduodenectomy in Elderly Patients. J. Laparoendosc. Adv. Surg. Tech. A 2020, 30, 797–802. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- The Jamovi Project. Jamovi, version 2.3; The Jamovi Project: Sidney, Australia, 2022.
- Son, I.T.; Kim, J.Y.; Kim, M.J.; Kim, B.C.; Kang, B.M.; Kim, J.W. Clinical and oncologic outcomes of laparoscopic versus open surgery in elderly patients with colorectal cancer: A retrospective multicenter study. Int. J. Clin. Oncol. 2021, 26, 2237–2245. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–961. [Google Scholar] [CrossRef] [PubMed]
- Turrentine, F.E.; Wang, H.; Simpson, V.B.; Jones, R.S. Surgical risk factors, morbidity, and mortality in elderly patients. J. Am. Coll. Surg. 2006, 203, 865–877. [Google Scholar] [CrossRef]
- González-Senac, N.M.; Mayordomo-Cava, J.; Macías-Valle, A.; Aldama-Marín, P.; Majuelos González, S.; Cruz Arnés, M.L.; Jiménez-Gómez, L.M.; Vidán-Astiz, M.T.; Serra-Rexach, J.A. Colorectal Cancer in Elderly Patients with Surgical Indication: State of the Art, Current Management, Role of Frailty and Benefits of a Geriatric Liaison. Int. J. Environ. Res. Public Health 2021, 18, 6072. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, X.; Zhao, C.; Liu, Q.; Zhou, H.; Zheng, Z.; Zhou, Z.; Wang, X.; Liang, J. Laparoscopic vs. open colorectal cancer surgery in elderly patients: Short- and long-term outcomes and predictors for overall and disease-free survival. BMC Surg. 2019, 19, 137. [Google Scholar] [CrossRef]
- Hinoi, T.; Kawaguchi, Y.; Hattori, M.; Okajima, M.; Ohdan, H.; Yamamoto, S.; Hasegawa, H.; Horie, H.; Murata, K.; Yamaguchi, S.; et al. Laparoscopic versus open surgery for colorectal cancer in elderly patients: A multicenter matched case-control study. Ann. Surg. Oncol. 2015, 22, 2040–2050. [Google Scholar] [CrossRef] [PubMed]
- Miguchi, M.; Yoshimitsu, M.; Shimomura, M.; Kohashi, T.; Egi, H.; Ohdan, H.; Hirabayashi, N. Long-term outcomes of laparoscopic surgery in elderly patients with colorectal cancer: A single institutional matched case-control study. Asian J. Endosc. Surg. 2021, 14, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wu, M.; Chen, Y. Laparoscopic versus open surgery for elderly patients with colorectal cancer: A systematic review and meta-analysis of matched studies. ANZ J. Surg. 2022, 92, 2003–2017. [Google Scholar] [CrossRef]
- Utsumi, M.; Matsuda, T.; Yamashita, K.; Hasegawa, H.; Agawa, K.; Urakawa, N.; Kanaji, S.; Oshikiri, T.; Nakamura, T.; Kakeji, Y. Short-term and long-term outcomes after laparoscopic surgery for elderly patients with colorectal cancer aged over 80 years: A propensity score matching analysis. Int. J. Color. Dis. 2021, 36, 2519–2528. [Google Scholar] [CrossRef]






| Characteristic | Group A (N = 113, 83%) | Group B (N = 23, 17%) | p-Value |
|---|---|---|---|
| Sex | n.s. | ||
| Female | 65 (58%) | 14 (61%) | |
| Male | 48 (42%) | 9 (39%) | |
| Age at surgery | 84 (81, 87) | 86 (84, 90) | n.s. |
| Provenance | n.s. | ||
| Accident and Emergency | 26 (24%) | 7 (30%) | |
| Home | 66 (60%) | 7 (30%) | |
| Other ward/hospital | 18 (16%) | 8 (35%) | |
| Pharmacological treatment | |||
| Antiplatelets | 31 (28%) | 4 (17%) | n.s. |
| Anticoagulants | 21 (19%) | 10 (43%) | n.s. |
| Symptoms | |||
| Anemia | 53 (47%) | 9 (39%) | n.s. |
| Rectorrhagia | 28 (25%) | 6 (26%) | n.s. |
| Obstruction | 19 (17%) | 9 (39%) | n.s. |
| Weight loss | 16 (14%) | 1 (4.3%) | n.s. |
| Change in bowel habit | 27 (24%) | 6 (26%) | n.s. |
| Abdominal pain | 31 (28%) | 3 (13%) | n.s. |
| Hemoglobin (g/L) | 107 (94, 119) | 104 (92, 114) | n.s. |
| Type of operation | n.s. | ||
| Anterior rectal resection + TME | 11 (9.7%) | 0 (0%) | |
| Miles | 5 (4.4%) | 0 (0%) | |
| Left flexure resection | 3 (2.7%) | 1 (4.3%) | |
| Left hemicolectomy | 22 (19%) | 2 (8.7%) | |
| Other (palliation) | 5 (4.4%) | 1 (4.3%) | |
| Right hemicolectomy | 42 (37%) | 7 (30%) | |
| Extended right hemicolectomy | 11 (9.7%) | 3 (13%) | |
| Sigmoid resection (partial TME) | 10 (8.8%) | 5 (22%) | |
| Subtotal colectomy | 0 (0%) | 2 (8.7%) | |
| Transverse colon resection | 4 (3.5%) | 2 (8.7%) | |
| Length of stay (days) | 10 (7, 15) | 14 (11, 18) | 0.04 |
| Complications (Clavien–Dindo > 2) | |||
| Medical | 20 (18%) | 6 (26%) | n.s. |
| Surgical | 17 (15%) | 1 (4.3%) | n.s. |
| Anastomotic leak | 9 (8.0%) | 0 (0%) | n.s. |
| 30-day outcome | n.s. | ||
| Dead | 8 (7.1%) | 5 (22%) | |
| Discharged | 98 (88%) | 16 (70%) | |
| Still hospitalized | 6 (5.4%) | 2 (8.7%) | |
| pT stage (N = 127) | n.s. | ||
| T1 | 10 (9.3%) | 0 (0%) | |
| T2 | 9 (8.4%) | 3 (15%) | |
| T3 | 71 (66%) | 9 (45%) | |
| T4a | 13 (12%) | 4 (20%) | |
| T4b | 4 (3.7%) | 4 (20%) | |
| Harvested lymph nodes | 19 (15, 26) | 14 (11, 22) | n.s. |
| Microsatellite instability (N = 127) | 28 (25%) | 5 (22%) | n.s. |
| Adjuvant chemotherapy (N = 47) | 5 (12%) | 0 (0%) | n.s. |
| Surgical Complications (Clavien–Dindo >2) | Group A (N = 113) | Group B (N = 23) |
|---|---|---|
| Anastomotic leak | 9 | 0 |
| Intraluminal bleeding | 2 | 0 |
| Hemorrhage | 2 | 0 |
| Abdominal collections | 3 | 1 |
| Bowel obstruction | 1 | 0 |
| Total | 17 (15%) | 1 (4.3%) |
| p = n.s. | ||
| Medical Complications (Clavien–Dindo > 2) | Group A (N = 113) | Group B (N = 23) |
| Ileus | 6 | 2 |
| Pneumonia/respiratory failure | 5 | 1 |
| Heart failure | 7 | 2 |
| AKI | 2 | 1 |
| Total | 20 (18%) | 6 (26%) |
| p= n.s. | ||
| Surgical Complications (Clavien–Dindo >2) | Group A (N = 113) | Group C (N = 269) |
| Anastomotic leak | 9 | 10 |
| Intraluminal bleeding | 2 | 2 |
| Hemorrhage | 2 | 2 |
| Abdominal collections | 3 | 2 |
| Bowel obstruction | 1 | 4 |
| Ureteral leak | - | 1 |
| Pancreatic leak | - | 1 |
| Vaginal leak | - | 1 |
| Total | 17 (15%) | 23 (8.6%) |
| p = n.s. | ||
| Medical Complications (Clavien–Dindo >2) | Group A (N = 113) | Group C (N = 269) |
| Ileus | 6 | 4 |
| Pneumonia/respiratory failure | 5 | 3 |
| Heart failure | 7 | 5 |
| Acute kidney injury | 2 | 1 |
| Non-surgical sepsis | - | 3 |
| Other (medical) | - | 2 |
| Total | 20 (18%) | 18 (6.7%) |
| p = 0.003 | ||
| Characteristic | Group A (N = 113, 30%) | Group C (N = 269, 70%) | p-Value |
|---|---|---|---|
| Sex | n.s. | ||
| Female | 65 (58%) | 116 (43%) | |
| Male | 48 (42%) | 153 (57%) | |
| Age at surgery | 84 (81, 87) | 65 (57, 71) | <0.001 |
| Provenance | <0.001 | ||
| Accidents and Emergency | 26 (24%) | 18 (6.8%) | |
| Home | 66 (60%) | 238 (89%) | |
| Other ward/hospital | 18 (16%) | 9 (3.4%) | |
| Charlson Comorbidity Index | 7 (6, 8) | 5 (4, 6) | <0.001 |
| Pharmacological treatment | |||
| Antiplatelets | 31 (28%) | 39 (15%) | 0.003 |
| Anticoagulants | 21 (19%) | 22 (8.4%) | 0.004 |
| Symptoms | |||
| Anemia | 53 (47%) | 42 (16%) | <0.001 |
| Rectorrhagia | 28 (25%) | 66 (25%) | n.s. |
| Obstruction | 19 (17%) | 8 (3.1%) | <0.001 |
| Weight loss | 16 (14%) | 17 (6.6%) | n.s. |
| Change in bowel habit | 27 (24%) | 29 (11%) | 0.001 |
| Abdominal pain | 31 (28%) | 42 (16%) | n.s. |
| Hemoglobin (g/L) | 107 (94, 119) | 131 (118, 142) | <0.001 |
| Surgical treatment | n.s. | ||
| Anterior rectal resection + TME | 11 (9.7%) | 46 (17%) | |
| Miles | 5 (4.4%) | 15 (5.6%) | |
| Left flexure resection | 3 (2.7%) | 5 (1.9%) | |
| Left hemicolectomy | 22 (19%) | 73 (27%) | |
| Other (palliation) | 5 (4.4%) | 2 (0.7%) | |
| Tight hemicolectomy | 42 (37%) | 79 (29%) | |
| Extended right hemicolectomy | 11 (9.7%) | 22 (8.2%) | |
| Sigmoid resection (partial TME) | 10 (8.8%) | 17 (6.3%) | |
| Subtotal colectomy | 0 (0%) | 5 (1.9%) | |
| Transverse colon resection | 4 (3.5%) | 5 (1.9%) | |
| Conversion to open | 17 (15%) | 14 (5.2%) | 0.003 |
| Length of stay | 10 (7, 15) | 8 (6, 10) | <0.001 |
| Complications (Clavien–Dindo > 2) | |||
| Medical | 20 (18%) | 18 (6.7%) | 0.003 |
| Surgical | 17 (15%) | 23 (8.6%) | n.s. |
| Anastomotic leak | 9 (8.0%) | 10 (3.7%) | n.s. |
| 30-day outcome | 0.001 | ||
| Dead | 8 (7.1%) | 1 (0.4%) | |
| Discharged | 98 (88%) | 257 (96%) | |
| Still hospitalized | 6 (5.4%) | 9 (3.4%) | |
| pT stage (N = 363) | n.s. | ||
| T0 | 0 (0%) | 9 (3.5%) | |
| T1 | 10 (9.3%) | 58 (22%) | |
| T2 | 9 (8.4%) | 44 (17%) | |
| T3 | 71 (66%) | 117 (46%) | |
| T4a | 13 (12%) | 24 (9.4%) | |
| T4b | 4 (3.7%) | 5 (2.0%) | |
| Harvested lymph nodes | 19 (15, 26) | 18 (13, 25) | n.s. |
| Microsatellite instability (N = 363) | 28 (25%) | 35 (13%) | 0.005 |
| Adjuvant chemotherapy (N = 187) | 5 (12%) | 55 (38%) | 0.002 |
| Conversion to Open Surgery | No | Yes | OR (Univariable) | OR (Multivariable) | ||
|---|---|---|---|---|---|---|
| Age at surgery | Mean (SD) | 69.4 (12.6) | 77.4 (13.4) | 1.06 (1.02–1.10, p = 0.001) | ||
| Charlson Comorbidity Index | Mean (SD) | 5.5 (2.1) | 6.9 (2.2) | 1.31 (1.12–1.53, p = 0.001) | ||
| Obstruction | No | 325 (93.9) | 21 (6.1) | - | - | |
| Yes | 17 (63.0) | 10 (37.0) | 9.10 (3.63–22.21, p < 0.001) | 6.15 (2.29–15.92, p < 0.001) | ||
| Medical Complications (CD > 2) | No | Yes | OR (univariable) | OR (multivariable) | ||
| Age at surgery | Mean (SD) | 69.4 (12.8) | 75.6 (11.5) | 1.04 (1.01–1.08, p = 0.006) | ||
| ASA score | Mean (SD) | 2.4 (0.6) | 3.0 (0.6) | 6.68 (3.08–16.73, p < 0.001) | 5.54 (2.28–15.16, p < 0.001) | |
| Anemia | No | 258 (93.1) | 19 (6.9) | - | - | |
| Yes | 79 (82.3) | 17 (17.7) | 2.92 (1.44–5.90, p = 0.003) | |||
| Charlson Comorbidity Index | Mean (SD) | 5.5 (2.2) | 6.3 (2.1) | 1.15 (1.00–1.33, p = 0.054) | ||
| 30-day outcome | Discharged | Dead | Still hospitalized | OR (univariable) | OR (multivariable) | |
| Age at surgery | Mean (SD) | 69.4 (12.7) | 86.0 (5.5) | 73.4 (12.8) | 1.06 (1.02–1.10, p = 0.002) | |
| Medical complications (CD > 2) | No | 326 (94.8) | 4 (1.2) | 14 (4.1) | - | - |
| Yes | 29 (76.3) | 5 (13.2) | 4 (10.5) | 5.62 (2.24–13.40, p < 0.001) | 3.76 (1.39–9.68, p = 0.007) | |
| Surgical complications (CD > 2) | No | 327 (95.6) | 4 (1.2) | 11 (3.2) | - | - |
| Yes | 28 (70.0) | 5 (12.5) | 7 (17.5) | 9.34 (3.94–21.95, p < 0.001) | 7.28 (2.95–17.79, p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passuello, N.; Polese, L.; Ometto, G.; Grossi, U.; Mammano, E.; Vittadello, F.; Frasson, A.; Tessari, E.; Bartolotta, P.; Gregori, D.; et al. Outcomes of Laparoscopic Surgery in Very Elderly Patients with Colorectal Cancer: A Survival Analysis and Comparative Study. J. Clin. Med. 2023, 12, 7122. https://doi.org/10.3390/jcm12227122
Passuello N, Polese L, Ometto G, Grossi U, Mammano E, Vittadello F, Frasson A, Tessari E, Bartolotta P, Gregori D, et al. Outcomes of Laparoscopic Surgery in Very Elderly Patients with Colorectal Cancer: A Survival Analysis and Comparative Study. Journal of Clinical Medicine. 2023; 12(22):7122. https://doi.org/10.3390/jcm12227122
Chicago/Turabian StylePassuello, Nicola, Lino Polese, Giulia Ometto, Ugo Grossi, Enzo Mammano, Fabrizio Vittadello, Alvise Frasson, Emanuela Tessari, Patrizia Bartolotta, Dario Gregori, and et al. 2023. "Outcomes of Laparoscopic Surgery in Very Elderly Patients with Colorectal Cancer: A Survival Analysis and Comparative Study" Journal of Clinical Medicine 12, no. 22: 7122. https://doi.org/10.3390/jcm12227122
APA StylePassuello, N., Polese, L., Ometto, G., Grossi, U., Mammano, E., Vittadello, F., Frasson, A., Tessari, E., Bartolotta, P., Gregori, D., & Sarzo, G. (2023). Outcomes of Laparoscopic Surgery in Very Elderly Patients with Colorectal Cancer: A Survival Analysis and Comparative Study. Journal of Clinical Medicine, 12(22), 7122. https://doi.org/10.3390/jcm12227122