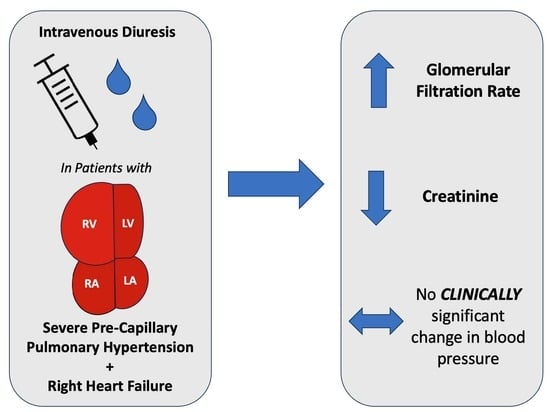
Intravenous Diuresis in Severe Precapillary Pulmonary-Hypertension-Related Right Heart Failure: Effects on Renal Function and Blood Pressure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Objectives
2.2. Study Design
2.3. Patient Selection
2.4. Baseline Characteristics Data Collection
2.5. Outcomes and Measures of Clinical Response
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Echocardiographic and Hemodynamic Data
| Mean ± SD | Normal Values | |
|---|---|---|
| Heart Rate (beats/min) | 80.2 ± 14.0 | 60−100 beats/min |
| SBP (mmHg) | 119.0 ± 24.2 | 90−140 mmHg |
| DBP (mmHg) | 69.2 ± 13.7 | 60−90 mmHg |
| MAP (mmHg) | 88.5 ± 17.3 | 70−105 mmHg |
| RA (mmHg) | 11.8 ± 5.5 | 2−6 mmHg |
| RVSP (mmHg) | 78.9 ± 16.5 | 15−25 mmHg |
| RVDP (mmHg) | 15.2 ± 8.1 | 0−8 mmHg |
| PASP (mmHg) | 70.0 ± 16.6 | 15−25 mmHg |
| PADP (mmHg) | 32.8 ± 8.4 | 8−15 mmHg |
| Mean PA (mmHg) | 49.9 ± 10.9 | 9−18 mmHg |
| PCWP (mmHg) | 10.1 ±3.9 | 6−12 mmHg |
| RA: PCWP | 1.3 ± 0.7 | ≤0.5 |
| CO (L/min) | 3.8 ± 1.2 | 4.0−8.0 L/min |
| CI (L/min/m2) | 2.1 ± 0.6 | 2.5−4 L/min/m2 |
| PVR (Woods Units) | 11.7 ± 5.7 | <2 WU |
| SVR (dynes/s/cm−5) | 1736 ± 824 | 800−1200 dynes/s/cm−5 |
3.3. Admission Characteristics
3.4. Renal Function and Hemodynamics
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. Evaluation and Management of Right-Sided Heart Failure. A Scientific Statement from the American Heart Association. Circulation 2018, 137, 578–622. [Google Scholar] [CrossRef]
- Alam, S.; Palevsky, H.I. Standard Therapies for Pulmonary Arterial Hypertension. Clin. Chest Med. 2007, 28, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Kakouros, N.; Cokkinos, D.V. Right ventricular myocardial infarction: Pathophysiology, diagnosis, and management. Postgrad. Med. J. 2010, 86, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Namana, V.; Satish Gupta, S.; Abbasi, A.A.; Raheja, H.; Shani, J.; Hollander, G. Right ventricular infarction. Cardiovasc. Revasc. Med. 2018, 19, 43–50. [Google Scholar] [CrossRef]
- Vaidy, A.; O’corragain, O.; Vaidya, A. Diagnosis and Management of Pulmonary Hypertension and Right Ventricular Failure in the Cardiovascular Intensive Care Unit. Crit. Care Clin. 2023. [Google Scholar] [CrossRef]
- Inampudi, C.; Tedford, R.J.; Hemnes, A.R.; Hansmann, G.; Bogaard, H.-J.; Koestenberger, M.; Lang, I.M.; Brittain, E.L. Treatment of right ventricular dysfunction and heart failure in pulmonary arterial hypertension. Cardiovasc. Diagn. Ther. 2020, 10, 1659. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Menachem, J.N.; Felker, G.M.; Patel, C.B. Right Atrial to Pulmonary Capillary Wedge Pressure Ratio Is Not Associated with Failure of Optimal Medical Management in the INTERMACS 4-5 Population. J. Heart Lung Transplant. 2013, 32, S22. [Google Scholar] [CrossRef]
- Maron, B.A.; Kovacs, G.; Vaidya, A.; Bhatt, D.L.; Nishimura, R.A.; Mak, S.; Guazzi, M.; Tedford, R.J. Cardiopulmonary Hemodynamics in Pulmonary Hypertension and Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 2671–2681. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Soliman, O.I.; Akin, S.; Muslem, R.; Boersma, E.; Manintveld, O.C.; Krabatsch, T.; Gummert, J.F.; de By, T.M.M.H.; Bogers, A.J.J.C.; Zijlstra, F.; et al. Derivation and Validation of a Novel Right-Sided Heart Failure Model After Implantation of Continuous Flow Left Ventricular Assist Devices. Circulation 2017, 137, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, G.M.; Ciarka, A.; Biniecka, M.; Ceranowicz, P. Right Heart Catheterization-Background, Physiological Basics, and Clinical Implications. J. Clin. Med. 2019, 8, 1331. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Brisco, M.A.; Turner, J.M.; Spatz, E.S.; Bellumkonda, L.; Parikh, C.R.; Tang, W.W. Loop diuretic efficiency a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ. Heart Fail. 2014, 7, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Badesch, D.B.; Abman, S.H.; Ahearn, G.S.; Barst, R.J.; McCrory, D.C.; Simonneau, G.; McLaughlin, V.V. Medical therapy for pulmonary arterial hypertension *: ACCP evidence-based clinical practice guidelines. Chest 2004, 126, 35S–62S. [Google Scholar] [CrossRef]
- Fuso, L.; Baldi, F.; di Perna, A. Therapeutic Strategies in Pulmonary Hypertension. Front. Pharmacol. 2011, 2, 21. [Google Scholar] [CrossRef]
- Goldstein, J.A. Right Heart Ischemia: Pathophysiology, Natural History, and Clinical Management. Prog. Cardiovasc. Dis. 1998, 40, 325. [Google Scholar] [CrossRef]
- Inohara, T.; Kohsaka, S.; Fukuda, K.; Menon, V. The challenges in the management of right ventricular infarction. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 226–234. [Google Scholar] [CrossRef]
- Zamanian, R.T.; Haddad, F.; Doyle, R.L.; Weinacker, A.B. Management strategies for patients with pulmonary hypertension in the intensive care unit. Crit. Care Med. 2007, 35, 2037–2050. [Google Scholar] [CrossRef]
- Ventetuolo, C.E.; Klinger, J.R. Management of Acute Right Ventricular Failure in the Intensive Care Unit. Ann. Am. Thorac. Soc. 2014, 11, 811. [Google Scholar] [CrossRef]
- Chin, K.M.; Rubin, L.J. Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2008, 51, 1527–1538. [Google Scholar] [CrossRef]
- Forfia, P.R.; Vaidya, A.; Wiegers, S.E. Pulmonary heart disease: The heart-lung interaction and its impact on patient phenotypes. Pulm. Circ. 2013, 3, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Arkles, J.S.; Opotowsky, A.R.; Ojeda, J.; Rogers, F.; Liu, T.; Prassana, V.; Marzec, L.; Palevsky, H.I.; Ferrari, V.A.; Forfia, P.R. Shape of the Right Ventricular Doppler Envelope Predicts Hemodynamics and Right Heart Function in Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2011, 183, 268–276. [Google Scholar] [CrossRef]
- Mentz, R.J.; Kjeldsen, K.; Rossi, G.P.; Voors, A.A.; Cleland, J.G.; Anker, S.D.; Gheorghiade, M.; Fiuzat, M.; Rossignol, P.; Zannad, F.; et al. Decongestion in acute heart failure. Eur. J. Heart Fail. 2014, 16, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; van Deursen, V.M.; Navis, G.; Voors, A.A.; van Veldhuisen, D.J.; Hillege, H.L. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. Coll. Cardiol. 2009, 53, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef]
- Testani, J.M.; Coca, S.G.; McCauley, B.D.; Shannon, R.P.; Kimmel, S.E. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur. J. Heart Fail. 2011, 13, 877–884. [Google Scholar] [CrossRef]


| Demographics | Mean ± SD or N (%) |
|---|---|
| Age, y | 64.7 ± 14.5 |
| Sex | |
| Men | 10 (16.1) |
| Women | 52 (83.9) |
| Race | |
| White | 38 (61.2) |
| Black | 16 (25.8) |
| Hispanic | 8 (12.9) |
| Other | 10 (16.1) |
| Body Mass Index (kg/m2) | 28.5 ± 8.1 |
| NYHA Functional Class (noted closest to admission) | |
| I | 2 (3.2) |
| II | 5 (8.1) |
| III | 33 (53.2) |
| IV | 11 (17.7) |
| Other Medications | |
| ACE/ARB | 8 (12.9) |
| MRA | 17 (27.4) |
| CCB | 4 (6.5) |
| Beta-blocker | 21 (33.9) |
| Statin | 26 (41.9) |
| Immunosuppressant | 5 (8.1) |
| Co-morbidities | |
| Hypertension | 31 (50) |
| Hyperlipidemia | 9 (14.5) |
| Diabetes Mellitus | 15 (24.2) |
| Chronic Kidney Disease | 10 (16.1) |
| Chronic Obstructive Pulmonary Disease | 16 (25.8) |
| Coronary Artery Disease | 12 (19.4) |
| Atrial arrhythmias | 12 (19.4) |
| Autoimmune Disease | 23 (37.1) |
| Diagnosis-PH WHO Group | |
|---|---|
| PAH (Group 1) | 56 (90.3) |
| PAH (Group 3) | 6 (9.7) |
| PH Medical Regimens on Admission | |
| PDE5 inhibitor | 44 (71) |
| Prostacyclin | 30 (48.4) |
| Endothelin receptor antagonist | 27 (43.5) |
| sGC Stimulator | 8 (12.8) |
| Single | 12 (19.3) |
| Dual | 11 (17.7) |
| Triple | 22 (35.4) |
| No PH Medical Therapy On Admission | 17 (27.4) |
| Group 1 | 14 (22.5) |
| Group 3 | 3 (4.8) |
| No PH Medical Therapy On Discharge | 2 (3.2) |
| Group 1 | 0 |
| Group 3 | 2 (3.2) |
| Echocardiographic Parameters | ||||
|---|---|---|---|---|
| Ejection Fraction (%) | 60.9 ± 8.0 | |||
| PASP (mmHg) | 79.2 ± 22.2 | |||
| TAPSE (cm) | 1.4 ± 0.4 | |||
| Right Atrial Size | Normal 2 (3) | Dilated 60 (97) | ||
| Left Atrial Size | Normal 50 (80.6) | Dilated 12 (19.4) | ||
| Ventricular Septal flattening | None 4 (6.4) | Mild 6 (9.7) | Moderate 23 (37.1) | Severe 29 (46.8) |
| RVOT Systolic Notching | Present 42 (67.7) | Absent 12 (19.4) | Not evaluated 8 (12.9) | |
| RV Size (Dilation) | Normal 1 (1.6) | Mild 3 (4.8) | Moderate 17 (27.4) | Severe 41 (66.1) |
| RV Function (Degree of Dysfunction) | Normal 2 (3.2) | Mild 3 (4.8) | Moderate 24 (38.7) | Severe 33 (53.2) |
| Tricuspid Regurgitation (Degree) | None 3 (4.8) | Mild 10 (16.1) | Moderate 29 (46.8) | Severe 20 (32.3) |
| Pericardial Effusion (size) | None 33 (53.2) | Mild 19 (30.6) | Moderate 7 (11.3) | Severe 3 (4.8) |
| Admission Characteristics | |||
|---|---|---|---|
| Duration (days) | 10.1 ± 6.4 | ||
| New PH Diagnosis on Admission | 14 (22.5) | ||
| 30 day readmission rate | 8 (12.9) | ||
| Total Liters Diuresed (L) | 13.3 ± 7.7 | ||
| Weight Change (kg) | −7.0 ± 6.7 | ||
| Mean highest daily diuretic dose (mg, Furosemide equivalents) | Median 240 (Min 20, Max 3840) | ||
| Diuretics Used | Number of Patients | ||
| Furosemide | 25 (40) | ||
| Bumetanide | 41 (66) | ||
| Torsemide | 2 (3) | ||
| Metolazone/Chlorothiazide | 5 (8) | ||
| Pre and Post Diuresis (Entire Cohort) | Admission | Discharge | p Value |
| Systolic Blood Pressure (mmHg) | 114 ± 20.6 | 106 ± 15.7 | p < 0.05 |
| Diastolic Blood Pressure (mmHg) | 71.1 ± 13.5 | 64.9 ± 9.8 | p < 0.05 |
| Mean Arterial Blood Pressure (mmHg) | 85.6 ± 14.5 | 78.5 ± 10.9 | p < 0.05 |
| Heart Rate (beats/min) | 86.8 ± 16.1 | 79.7 ± 13.0 | p < 0.05 |
| Creatinine (mg/dL) | 1.4 ± 0.5 | 1.2 ± 0.4 | p < 0.05 |
| GFR (mL/min/1.73 m2) | 47.3 ± 12.1 | 50.3 ± 11.1 | p < 0.05 |
| Serum Sodium (mmol/L) | 137.3 ± 5.2 | 137 ± 3.9 | p < 0.3 |
| Jugular Venous Pressure (cm H2O) | 15.8 ± 3.8 | 8.7 ± 1.8 | p < 0.05 |
| In Patients Receiving > 1000 mg Furosemide equivalents daily | Admission | Discharge | p Value |
| Mean Arterial Blood Pressure (mmHg) | 87.3 ± 13.5 | 81.3 ± 12.4 | p < 0.1 |
| Creatinine (mg/dL) | 1.8 ± 0.4 | 1.4 ± 0.5 | p < 0.05 |
| Glomerular Filtration Rate (mL/min/1.73 m2) | 37.8 ±9.9 | 46.1 ± 14.1 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labrada, L.; Romero, C.; Sadek, A.; Belardo, D.; Raza, Y.; Forfia, P. Intravenous Diuresis in Severe Precapillary Pulmonary-Hypertension-Related Right Heart Failure: Effects on Renal Function and Blood Pressure. J. Clin. Med. 2023, 12, 7149. https://doi.org/10.3390/jcm12227149
Labrada L, Romero C, Sadek A, Belardo D, Raza Y, Forfia P. Intravenous Diuresis in Severe Precapillary Pulmonary-Hypertension-Related Right Heart Failure: Effects on Renal Function and Blood Pressure. Journal of Clinical Medicine. 2023; 12(22):7149. https://doi.org/10.3390/jcm12227149
Chicago/Turabian StyleLabrada, Lyana, Carlos Romero, Ahmed Sadek, Danielle Belardo, Yasmin Raza, and Paul Forfia. 2023. "Intravenous Diuresis in Severe Precapillary Pulmonary-Hypertension-Related Right Heart Failure: Effects on Renal Function and Blood Pressure" Journal of Clinical Medicine 12, no. 22: 7149. https://doi.org/10.3390/jcm12227149
APA StyleLabrada, L., Romero, C., Sadek, A., Belardo, D., Raza, Y., & Forfia, P. (2023). Intravenous Diuresis in Severe Precapillary Pulmonary-Hypertension-Related Right Heart Failure: Effects on Renal Function and Blood Pressure. Journal of Clinical Medicine, 12(22), 7149. https://doi.org/10.3390/jcm12227149