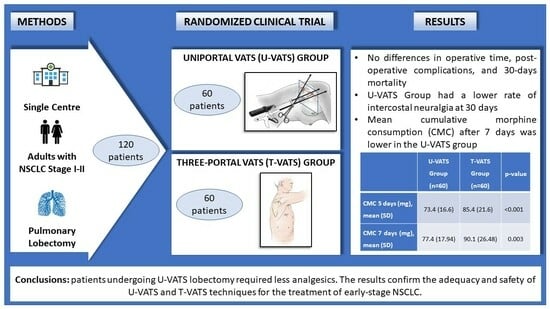
Pulmonary Lobectomy for Early-Stage Lung Cancer with Uniportal versus Three-Portal Video-Assisted Thoracic Surgery: Results from a Single-Centre Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Endpoints
2.3. Inclusion and Exclusion Criteria
- Age < 18 or >80 years;
- Clinical stage > II;
- Clinical T3 disease;
- ASA score > 3;
- Patients evaluated for sublobar resection, bilobectomy, sleeve lobectomy, or pneumonectomy;
- Previous thoracic surgery, induction therapy, or chest radiotherapy
- Connective tissue diseases, peripheral vascular diseases, major organ failure (kidney, liver, heart);
- Severe chronic obstructive pulmonary disease (COPD), asthma, or interstitial lung disease;
- Chronic use of analgesics or corticosteroids;
- Lack of informed consent.
- Massive pleural adhesions;
- Conversion to open surgery or supplementary trocar.
2.4. Surgical Techniques
2.5. Postoperative Care
2.6. Pain Evaluation and Analgesic Protocol
- NRS < 4: infusion rate was decreased by 0.125 mg/h;
- NRS = 4: infusion rate was not altered;
- NRS > 4: infusion rate was increased by 0.125 mg/h;
- NRS > 6: 1 mg bolus of morphine was administered.
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality World-wide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of stage I and II nonsmall cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. 5), e278S–e313S. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aggarwal, C.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 3.2019. 2019. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 10 August 2023).
- Detterbeck, F. Thoracoscopic versus open lobectomy debate: The pro argument. Thorac. Surg. Sci. 2009, 6, Doc04. [Google Scholar] [CrossRef] [PubMed]
- Falcoz, P.E.; Puyraveau, M.; Thomas, P.A.; Decaluwe, H.; Hürtgen, M.; Petersen, R.H.; Decaluwe, H. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: A propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur. J. Cardio-Thorac. Surg. 2016, 49, 602–609. [Google Scholar] [CrossRef]
- Yang, C.F.J.; Kumar, A.; Klapper, J.A.; Hartwig, M.G.; Tong, B.C.; Harpole, D.H., Jr.; D’Amico, T.A. A National Analysis of Long-term Survival Following Thoracoscopic Versus Open Lobectomy for Stage I Non-small-cell Lung Cancer. Ann. Surg. 2019, 269, 163–171. [Google Scholar] [CrossRef]
- Zhang, R.; Ferguson, M.K. Video-assisted versus open lobectomy in patients with compromised lung function: A literature review and meta-analysis. PLoS ONE 2015, 10, e0124512. [Google Scholar] [CrossRef]
- Bendixen, M.; Jørgensen, O.D.; Kronborg, C.; Andersen, C.; Licht, P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet Oncol. 2016, 17, 836–844. [Google Scholar] [CrossRef]
- Lim, E.; Batchelor, T.J.; Dunning, J.; Shackcloth, M.; Anikin, V.; Naidu, B.; Rogers, C.A. Video-Assisted Thoracoscopic or Open Lobectomy in Early-Stage Lung Cancer. NEJM Evid. 2022, 1, EVIDoa2100016. [Google Scholar] [CrossRef]
- Hansen, H.J.; Petersen, R.H. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach—The Copenhagen experience. Ann. Cardiothorac. Surg. 2012, 1, 70–76. [Google Scholar]
- Tosi, D.; Nosotti, M.; Bonitta, G.; Mazzucco, A.; Righi, I.; Mendogni, P.; Crisci, R. Uniportal and threeportal video-assisted thoracic surgery lobectomy: Analysis of the Italian video-assisted thoracic surgery group database. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 714–721. [Google Scholar] [CrossRef]
- Gonzalez, D.; Paradela, M.; Garcia, J.; dela Torre, M. Single-port video-assisted thoracoscopic lobectomy. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Bertolaccini, L.; Batirel, H.; Brunelli, A.; Gonzalez-Rivas, D.; Ismail, M.; Ucar, A.M.; Rocco, G. Uniportal video-assisted thoracic surgery lobectomy: A consensus report from the Uniportal VATS Interest Group (UVIG) of the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardiothorac. Surg. 2019, 56, 224–229. [Google Scholar] [PubMed]
- Nosotti, M.; Musso, V. A different video-assisted thoracoscopic approach for every patient or for every surgeon? Future Oncol. 2020, 16, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.P.; Ogunnaike, B.O. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol. Clin. N. Am. 2005, 23, 21–36. [Google Scholar]
- Harris, C.G.; James, R.S.; Tian, D.H.; Yan, T.D.; Doyle, M.P.; Gonzalez-Rivas, D.; Cao, C. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann. Cardiothorac. Surg. 2016, 5, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Perna, V.; Carvajal, A.F.; Torrecilla, J.A.; Gigirey, O. Uniportal video-assisted thoracoscopic lobectomy versus other video-assisted thoracoscopic lobectomy techniques: A randomized study. Eur. J. Cardiothorac. Surg. 2016, 50, 411–415. [Google Scholar] [CrossRef]
- Ng, C.S.; MacDonald, J.K.; Gilbert, S.; Khan, A.Z.; Kim, Y.T.; Louie, B.E.; Fernando, H.C. Optimal approach to lobectomy for non-small cell lung cancer: Systemic review and meta-analysis. Innovations 2019, 14, 90–116. [Google Scholar] [CrossRef]
- Xiao, L.; Yank, V.; Ma, J. Algorithm for balancing both continuous and categorical covariates in randomized controlled trials. Comput. Methods Programs Biomed. 2012, 108, 11851190. [Google Scholar]
- Longrois, D.; Hoeft, A.; De Hert, S. 2014 European Society of Cardiology/European Society of Anaesthesiology guidelines on non-cardiac surgery: Cardiovascular assessment and management: A short explanatory statement from the European Society of Anaesthesiology members who participated in the European Task Force. Eur. J. Anaesthesiol. 2014, 31, 513–516. [Google Scholar]
- Falcoz, P.E.; Conti, M.; Brouchet, L.; Chocron, S.; Puyraveau, M.; Mercier, M.; Dahan, M. The Thoracic Surgery Scoring System (Thoracoscore): Risk model for in-hospital death in 15,183 patients requiring thoracic surgery. J. Thorac. Cardiovasc. Surg. 2007, 133, 325–332. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Karnofsky, D.A.; Burchenal, J.H. The clinical evaluation of chemotherapeutic agents in cancer. In Evaluation of Chemotherapeutic Agents; MacLeod, C.M., Ed.; Columbia University Press: New York, NY, USA, 1949; pp. 191–205. [Google Scholar]
- American Society of Anesthesiologists. ASA Physical Status Classification System. 2020. Available online: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system (accessed on 15 August 2023).
- Mendogni, P.; Mazzucco, A.; Palleschi, A.; Rosso, L.; Righi, I.; Carrinola, R.; Tosi, D. Uniportal and three-portal video-assisted thoracic surgery pulmonary lobectomy for early-stage lung cancer (UNIT trial): Study protocol of a single-center randomized trial. Trials 2021, 22, 163. [Google Scholar]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ. 2010, 1, 100–107. [Google Scholar]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; Waller, D.; Passlick, B.; Weder, W. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef]
- Nosotti, M.; Rosso, L.; Tosi, D.; Palleschi, A.; Mendogni, P.; Righi, I.; Santambrogio, L. Preventive analgesia in thoracic surgery: Controlled, randomized, double-blinded study. Eur. J. Cardio-Thorac. Surg. 2015, 48, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, M.; McKenzie, D. Pursuit of Balance: Randomization in Practice in Development Field Experiments; The World Bank: Washington, DC, USA, 2008; p. 4752. [Google Scholar]
- Stuart, E.A.; Lee, B.K.; Leacy, F.P. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J. Clin. Epidemiol. 2013, 66 (Suppl. 8), S84–S90.e1. [Google Scholar]
- Kahan, B.C.; White, I.R.; Edwards, M.; Harhay, M.O. Using modified intention-to-treat as a principal stratum estimator for failure to initiate treatment. Clin. Trials 2023, 20, 269–275. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 August 2023).
- Chow, S.; Shao, J.; Wang, H. Sample Size Calculations in Clinical Research, 2nd ed.; Chapman & Hall/CRC Biostatistics Series; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Louis, S.G.; Gibson, W.J.; King, C.L.; Veeramachaneni, N.K. Uniportal video-assisted thoracoscopic surgery (VATS) technique is associated with decreased narcotic usage over traditional VATS lobectomy. J. Vis. Surg. 2017, 3, 117. [Google Scholar]
- Scott, W.J.; Allen, M.S.; Darling, G.; Meyers, B.; Decker, P.A.; Putnam, J.B.; Malthaner, R.A. Video-assisted thoracic surgery versus open lobectomy for lung cancer: A secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J. Thorac. Cardiovasc. Surg. 2010, 139, 976–981; discussion 981–983. [Google Scholar] [CrossRef]
- Copay, A.G.; Subach, B.R.; Glassman, S.D.; Polly, D.W., Jr.; Schuler, T.C. Understanding the minimum clinically important difference: A review of concepts and methods. Spine J. 2007, 7, 541–546. [Google Scholar] [PubMed]
- Aiolfi, A.; Bonitta, G.; Tosi, D.; Rausa, E.; Nosotti, M.; Bona, D.; Bonavina, L. Comment on: Two decades of surgical randomized controlled trials: Worldwide trends in volume and methodological quality. Br. J. Surg. 2023, 110, 1556. [Google Scholar] [CrossRef] [PubMed]

| U-VATS Group (n = 60) | T-VATS Group (n = 60) | SMD | p-Value | |
|---|---|---|---|---|
| Age, years, mean (SD) | 66.7 (11.3) | 68.8 (8.7) | 0.21 | 0.26 |
| Level of education, n (%) A B C D | 5(8.3) 11 (18.3) 28 (46.7) 16 (26.7) | 2 (3.4) 18 (30.0) 29 (48.3) 11 (18.3) | 0.213 0.249 0.042 0.191 | 0.26 0.14 0.85 0.27 |
| Male, n (%) | 31 (51.7) | 30 (50.0) | 0.142 | 0.85 |
| Thoracoscore, mean (SD) | 2.0 (1.2) | 2.3 (1.3) | 0.242 | 0.19 |
| BMI, kg/m2, mean (SD) | 26.0 (3.9) | 25.6 (4.4) | 0.071 | 0.60 |
| FEV1 %, mean (SD) | 98.8 (16.9) | 101.7 (23.9) | 0.143 | 0.45 |
| FVC %, mean (SD) | 108.1 (23.1) | 113.4 (20.5) | 0.241 | 0.23 |
| FEV1/FVC %, mean (SD) | 74.0 (9.2) | 71.8 (10.3) | 0.233 | 0.21 |
| DLCO %, mean (SD) | 83.3 (18.4) | 79.2 (15.9) | 0.243 | 0.20 |
| DLCO/VA %, mean (SD) | 91.1 (16.1) | 87.6(17.4) | 0.211 | 0.29 |
| Charlson Comorbidity Index, mean (SD) | 4.7(1.8) | 5.0 (1.5) | 0.184 | 0.51 |
| Karnofsky Performance Status %, mean (SD) | 96.0 (5.3) | 94.8 (5.4) | 0.226 | 0.41 |
| ASA score, n (%) | ||||
| 2 | 38 (63.3) | 31 (51.7) | 0.22 | 0.49 |
| 3 | 22 (36.7) | 29 (48.3) | 0.22 | 0.20 |
| Right upper lobe lobectomy, n (%) | 20 (33.3) | 25 (41.7) | 0.19 | 0.34 |
| Middle lobe lobectomy, n (%) | 6 (10) | 5 (8.3) | 0.07 | 0.75 |
| Right lower lobe lobectomy, n (%) | 10 (16.7) | 9 (15.0) | 0.05 | 0.80 |
| Left upper lobe lobectomy, n (%) | 15 (25) | 13 (21.7) | 0.07 | 0.67 |
| Left lower lobe lobectomy, n (%) | 9 (15) | 8 (13.3) | 0.06 | 0.79 |
| U-VATS Group (n = 60) | T-VATS Group (n = 60) | p-Value | Difference in Means (CI) | |
|---|---|---|---|---|
| CMC 1 day (mg), mean (SD) | 30.4 (18.3) | 37.9 (20.5) | 0.037 | 7.5 (0.52–14.47) |
| CMC 2 days (mg), mean (SD) | 49.8 (19.5) | 62.9 (21.2) | <0.001 | 13.1 (5.82–20.37) |
| CMC 3 days (mg), mean (SD) | 65.2 (16.3) | 76.1 (20.2) | 0.002 | 10.9 (4.33–17.46) |
| CMC 5 days (mg), mean (SD) | 73.4 (16.6) | 85.4 (21.6) | <0.001 | 12.0 (5.04–19.00) |
| CMC 7 days (mg), mean (SD) | 77.4 (17.94) | 90.1 (26.48) | 0.003 | 12.7 (4.45–20.82) |
| U-VATS Group (n = 60) | T-VATS Group (n = 60) | p-Value a | |
|---|---|---|---|
| NRS POD 1, mean (SD) | 2.3 (1.2) | 2.1 (1.3) | 0.99 |
| NRS POD 2, mean (SD) | 1.9 (1.1) | 2.1 (1.1) | 0.99 |
| NRS POD 3, mean (SD) | 1.5 (1.2) | 1.5 (1.1) | 0.99 |
| NRS POD 4, mean (SD) | 1.0 (1.3) | 1.3 (1.3) | 0.99 |
| NRS POD 5, mean (SD) | 0.8 (1.2) | 1.0 (1.1) | 0.99 |
| NRS POD 6, mean (SD) | 0.5 (0.9) | 0.8 (1.0) | 0.28 |
| NRS POD 7, mean (SD) | 0.3 (0.6) | 0.5 (0.9) | 0.59 |
| U-VATS Group (n = 60) | T-VATS Group (n = 60) | p-Value | |
|---|---|---|---|
| Pleural adhesions, n (%) | 18 (30) | 21 (35) | 0.56 |
| Air leakage at the end of surgery, n (%) | 6 (10) | 10 (16.7) | 0.29 |
| Surgical time, minutes, mean (SD) | 232 (55) | 220 (56) | 0.25 |
| N° lymph nodes, mean (SD) | 14.8 (6.2) | 15.2 (6.6) | 0.69 |
| N° lymph node stations, mean (SD) | 6.2 (1.6) | 6.3 (1.0) | 0.84 |
| R0 resection, n (%) | 60 (100) | 60 (100) | NS |
| Postoperative air leaks duration, days, mean (SD) | 1.2 (2.0) | 1.4 (2.3) | 0.49 |
| Chest drain dwelling time, days, mean (SD) | 4.0 (2.0) | 4.7 (2.4) | 0.06 |
| Hospital stay, days, mean (SD) | 6.3 (2.6) | 6.6 (2.4) | 0.47 |
| Prolonged air leaks, n (%) | 5 (8.3) | 5 (8.3) | 0.99 |
| Surgical site infection, n (%) | 0 (0.0) | 0 (0.0) | 0.99 |
| Pulmonary infection, n (%) | 4 (6.7) | 4 (6.7) | 0.99 |
| Arrythmia, n (%) | 3 (5.0) | 2 (3.3) | 0.65 |
| Other complications, n (%) | 0 (0.0) | 1 (1.7) | 0.31 |
| 30 days intercostal neuralgia, n (%) | 3 (5.0) | 9 (15.0) | 0.07 |
| 30 days mortality, n (%) | 0 (0.0) | 0 (0.0) | 0.99 |
| 7 POD | U-VATS Group (n = 50) | T-VATS Group (n = 46) | p-Value ** |
|---|---|---|---|
| FEV1 %, mean (SD) | 70.4 (16.1) | 71.6 (16.3) | 0.84 |
| FVC %, mean (SD) | 75.3 (16.2) | 77.1 (17.0) | 0.06 |
| FEV1/FVC % mean (SD) | 74.0 (7.2) | 73.2 (8.1) | 0.99 |
| 30 POD | U-VATS Group (n = 50) | T-VATS Group (n = 39) | p-Value ** |
| FEV1 %, mean (SD) | 78.0 (17.3) | 78.0 (18.1) | 0.95 |
| FVC %, mean (SD) | 83.0 (17.2) | 86.0 (18.0) | 0.82 |
| FEV1/FVC % mean (SD) | 75.0 (6.5) | 72.0 (8.9) | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosi, D.; Mazzucco, A.; Musso, V.; Bonitta, G.; Rosso, L.; Mendogni, P.; Righi, I.; Carrinola, R.; Damarco, F.; Palleschi, A. Pulmonary Lobectomy for Early-Stage Lung Cancer with Uniportal versus Three-Portal Video-Assisted Thoracic Surgery: Results from a Single-Centre Randomized Clinical Trial. J. Clin. Med. 2023, 12, 7167. https://doi.org/10.3390/jcm12227167
Tosi D, Mazzucco A, Musso V, Bonitta G, Rosso L, Mendogni P, Righi I, Carrinola R, Damarco F, Palleschi A. Pulmonary Lobectomy for Early-Stage Lung Cancer with Uniportal versus Three-Portal Video-Assisted Thoracic Surgery: Results from a Single-Centre Randomized Clinical Trial. Journal of Clinical Medicine. 2023; 12(22):7167. https://doi.org/10.3390/jcm12227167
Chicago/Turabian StyleTosi, Davide, Alessandra Mazzucco, Valeria Musso, Gianluca Bonitta, Lorenzo Rosso, Paolo Mendogni, Ilaria Righi, Rosaria Carrinola, Francesco Damarco, and Alessandro Palleschi. 2023. "Pulmonary Lobectomy for Early-Stage Lung Cancer with Uniportal versus Three-Portal Video-Assisted Thoracic Surgery: Results from a Single-Centre Randomized Clinical Trial" Journal of Clinical Medicine 12, no. 22: 7167. https://doi.org/10.3390/jcm12227167
APA StyleTosi, D., Mazzucco, A., Musso, V., Bonitta, G., Rosso, L., Mendogni, P., Righi, I., Carrinola, R., Damarco, F., & Palleschi, A. (2023). Pulmonary Lobectomy for Early-Stage Lung Cancer with Uniportal versus Three-Portal Video-Assisted Thoracic Surgery: Results from a Single-Centre Randomized Clinical Trial. Journal of Clinical Medicine, 12(22), 7167. https://doi.org/10.3390/jcm12227167