An Artificial Intelligence System for Optimizing Radioactive Iodine Therapy Dosimetry
Abstract
:1. Introduction
2. Dosimetric Approaches for RAIT
- (a)
- Bone marrow dose limiting, originally introduced by Benua et al. in 1962 [19] and later by Leeper [20] and Benua and Leeper [21] and further improved with the introduction of the MIRD formulism (Medical Internal Radiation Dosimetry Committee of the Society of Nuclear Medicine). Bone marrow is the most radiosensitive tissue in the body and is commonly the dose-limiting one for radionuclide therapy [22]. The method is mainly concerned with the safety of the treatment and considers blood as a bone marrow surrogate [23]. This approach accepts a threshold of a blood-absorbed dose of 2 Gy (200 rad) to avoid myelotoxicity [24] and the generally accepted thresholds of a lesion-absorbed dose of 300 Gy for thyroid remnants and of 80 Gy for metastases. This method also takes into consideration the absorbed dose to the lungs, ensuring it remains below 30 Gy to avoid pulmonary fibrosis; this translates into a threshold of 4.4 GBq (120 mCi) as the whole-body retention at 48 h and of less than 3 GBq (80 mCi) for patients with iodine-avid diffuse lung metastases [21,24]. Based on this method, the activity in the whole body is measured using gamma camera imaging in conjugate views (anterior and posterior) following the administration of tracer activity. The frequency of the imaging scans and the time intervals for blood sampling are detailed in relevant publications [24] and usually involve imaging over a course of 4–5 days at time points of 2 h, 24 h, 48 h, 72 h, and 96 h post-administration, with some variations depending on the exact implementation. The activity in the blood is measured using serial blood sampling methods correlating with the imaging time points. Mathematical analysis is applied, usually through software, to integrate the various organ time–activity curves and to calculate the total absorbed dose of radiation to the blood [15]. The details of the formulism’s implementation are described in the MIRD pamphlets [25,26,27] and other relevant publications [11,22] and are beyond the scope of this manuscript. Software that implements the MIRD formulism was also developed [28].
- (b)
- The lesion-based approach aims to improve the efficacy of treatment planning by delivering minimum absorbed doses of 300 Gy for remnant thyroid tissue and 80 Gy for metastases [29]. These thresholds were originally based on data presented by Maxon et al. [30] and Maxon and Smith [31]. The value of 80 Gy was originally defined for the treatment of cervical lymph node metastases and is assumed to be accurate for distant metastases. Using this method, dosimetric analysis can be performed post-treatment on thyroid remnant tissue or on lesions to assess the effectiveness of the treatment dose by correlating with clinical results [13]. An advantage of this method is its ability to ablate remnants with lower activities [29]. Uptake and clearance of I-131 from identifiable thyroid remnants and/or metastatic lesions can be generally measured using modeling that can be incorporated into software, such as the well-known OLINDA/EXM model [32].
3. Efforts to Optimize and Simplify Dosimetry Protocols
4. Methods and Materials
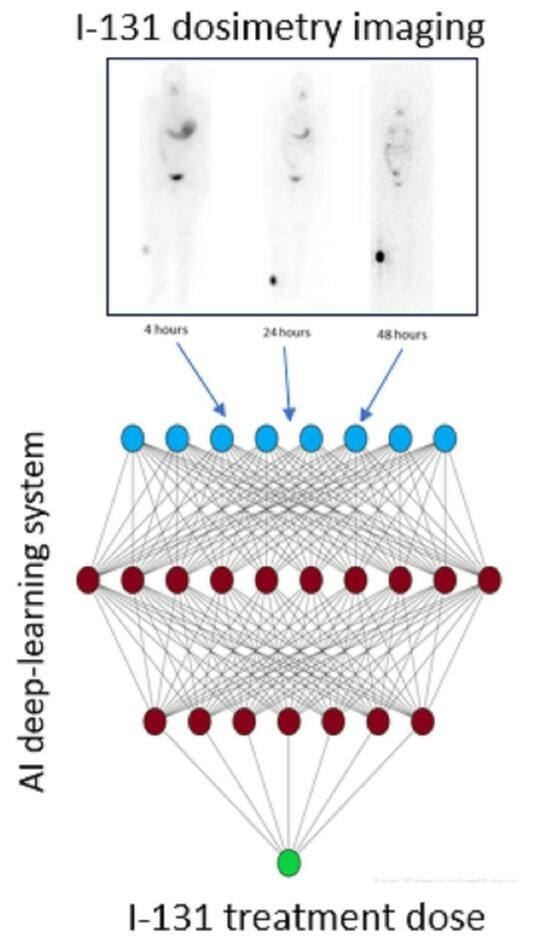
5. Artificial Intelligence System
6. Results
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.J.; Robeson, W.; Yoshida-Hay, M.; Zanzonico, P.B.; Leveque, F.; Bhargava, K.K.; Tronco, G.G.; Palestro, C.J. Alternative means of estimating 131I maximum permissible activity to treat thyroid cancer. J. Nucl. Med. 2017, 58, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Jemal, A.; Ward, E.M. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 2009, 115, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Fatholahi, L.; Tabeie, F.; Pashazadeh, A.M.; Javadi, H.; Assadi, M.; Asli, I.N. One size does not fit all: The merit of absorbed dose to the blood in 131I therapy for differentiated thyroid carcinoma. Health Phys. 2015, 108, 53–58. [Google Scholar] [CrossRef]
- Pacilio, M.; Conte, M.; Frantellizzi, V.; De Feo, M.S.; Pisani, A.R.; Marongiu, A.; Nuvoli, S.; Rubini, G.; Spanu, A.; De Vincentis, G. Personalized dosimetry in the context of radioiodine therapy for differentiated thyroid cancer. Diagnostics 2022, 12, 1763. [Google Scholar] [CrossRef]
- Luster, M.; Clarke, S.E.; Dietlein, M.; Lassmann, M.; Lind, P.; Oyen, W.J.G.; Tenvall, J.; Bombardieri, E. European Association of Nuclear Medicine guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1941–1959. [Google Scholar] [CrossRef]
- Gulec, S.A.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Draganescu, C.; Elisei, R.; Giovanella, L.; Grant, F.D.; Greenspan, B.; et al. A joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the European Thyroid Association, the Society of Nuclear Medicine and Molecular Imaging on current diagnostic and theranostic approaches in the management of thyroid cancer. Thyroid 2021, 31, 1009–1019. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Yang, R.; Zeng, W.; Peng, D.; Li, J.; Wang, H. Recent development of nuclear molecular imaging in thyroid cancer. BioMed Res. Int. 2018, 2018, 2149532. [Google Scholar] [CrossRef]
- Avram, A.M.; Giovanella, L.; Greenspan, B.; Lawson, S.A.; Luster, M.; Van Nostrand, D.; Peacock, J.G.; Ovcaricek, P.P.; Silberstein, E.; Tulchinsky, M.; et al. SNMMI procedure standard/EANM practice guideline for Nuclear Medicine evaluation and therapy of differentiated thyroid cancer: Abbreviated version. J. Nucl. Med. 2022, 63, 15N–35N. [Google Scholar]
- Ahn, B.C. Sodium iodide symporter for nuclear molecular imaging and gene therapy: From bedside to bench and back. Theranostics 2012, 2, 392–402. [Google Scholar] [CrossRef]
- Lassmann, M.; Reiners, C.; Luster, M. Dosimetry and thyroid cancer: The individual dosage of radioiodine. Endocr. Relat. Cancer 2010, 17, R161–R172. [Google Scholar] [CrossRef]
- Sawka, A.M.; Brierley, J.D.; Tsang, R.W.; Thabane, L.; Rotstein, L.; Gafni, A.; Straus, S.; Goldsteine, D.P. An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinol. Metab. Clin. N. Am. 2008, 37, 457–480. [Google Scholar] [CrossRef]
- Song, X.; Meng, Z.; Jia, Q.; Zhang, L.; Xu, K.; Tan, J.; Zhang, G.; Zheng, W.; Li, X.; Zhang, J. Different radioiodine dose for remnant thyroid ablation in patients with differentiated thyroid cancer. A meta-analysis. Clin. Nucl. Med. 2015, 40, 774–779. [Google Scholar] [CrossRef]
- Sisson, J.C.; Carey, J.E. Thyroid carcinoma with high levels of function: Treatment with 131I. J. Nucl. Med. 2001, 42, 975–983. [Google Scholar]
- Van Nostrand, D.; Atkins, F.; Moreau, S.; Aiken, M.; Kulkarni, K.; Wu, J.S.; Burman, K.D.; Wartofsky, L.; Haugen, B.R.; Alexander, E.K.; et al. Utility of the radioiodine whole-body retention at 48 hours for modifying empiric activity of I131-iodine for the treatment of metastatic well-differentiated thyroid carcinoma. Thyroid 2009, 19, 1093–1098. [Google Scholar] [CrossRef]
- Medvedec, M. Thyroid stunning in vivo and in vitro. Nucl. Med. Commun. 2005, 26, 731–735. [Google Scholar] [CrossRef]
- Besli, L.U.; Demir, M. Radiation dosimetry in thyroid cancer patients. In Thyroid Cancer—Advances in Diagnosis and Therapy; Intech: Rijeka, Croatia, 2016; Chapter 12; pp. 229–245. [Google Scholar] [CrossRef]
- Dorn, R.; Kopp, J.; Vogt, H.; Heidenreich, P.; Caroll, R.B.; Gulec, S. Dosimetry-guided radioactive iodine treatment in patients with metastatic differentiated thyroid cancer: Largest safe dose using a risk-adapted approach. J. Nucl. Med. 2003, 44, 451–456. [Google Scholar]
- Benua, R.S.; Cicale, N.R.; Sonenberg, M.; Rawson, R.W. The relation of radioiodine dosimetry to results and complications in the treatment of metastatic thyroid cancer. AJR 1962, 87, 171–182. [Google Scholar]
- Leeper, R.D. Thyroid cancer. Med. Clin. N. Am. 1985, 69, 1079–1096. [Google Scholar] [CrossRef]
- Benua, R.S.; Leeper, R.D. A method and rationale for treating metastatic thyroid carcinoma with the largest safe dose of I-131. In Frontiers in Thyroidology; Meideiros-Neto, G., Gaitan, E., Eds.; Plenum Medical Book: New York, NY, USA, 1986; Volume 2, pp. 1317–1321. [Google Scholar]
- Hindorf, C.; Glatting, G.; Chiesa, C.; Lindén, O.; Flux, G.; EANM Dosimetry Committee. EANM Dosimetry Committee guidelines for bone marrow and whole-body dosimetry. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1238–1250. [Google Scholar] [CrossRef]
- Sgouros, G. Blood and bone marrow dosimetry in radioiodine therapy of thyroid cancer. J. Nucl. Med. 2005, 46, 899–900. [Google Scholar] [PubMed]
- Lassmann, M.; Hänscheid, H.; Chiesa, C.; Hindorf, C.; Flux, G.; Luster, M. EANM Dosimetry Committee series on standard operational procedures for pre-therapeutic dosimetry I: Blood and bone marrow dosimetry in differentiated thyroid cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Loevinger, R.; Budinger, T.F.; Watson, E.E. MIRD PRIMER for Absorbed Dose Calculations, Revised Edition; The Society of Nuclear Medicine: Reston, VA, USA, 1991. [Google Scholar]
- Siegel, J.A.; Thomas, S.R.; Stubbs, J.B.; Stabin, M.G.; Hays, M.T.; Koral, K.F.; Robertson, J.S.; Howell, R.W.; Wessels, B.W.; Fisher, D.R.; et al. MIRD pamphlet no. 16: Techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J. Nucl. Med. 1999, 40, 37S–61S. [Google Scholar] [PubMed]
- Dewaraja, Y.K.; Ljungberg, M.; Green, A.J.; Zanzonico, P.B.; Frey, E.C. MIRD pamphlet no. 24: Guidelines for quantitative 131I SPECT in dosimetry applications. J. Nucl. Med. 2013, 54, 2182–2188. [Google Scholar] [CrossRef] [PubMed]
- Stabin, M.G. MIRDOSE: Personal computer software for internal dose assessment in nuclear medicine. J. Nucl. Med. 1996, 37, 538–546. [Google Scholar] [PubMed]
- Selcuk, A.N.; Toklu, T.; Beykan, S.; Karaaslan, S.I. Evaluation of the dosimetry approaches in ablation treatment of thyroid cancer. J. Appl. Clin. Med. Phys. 2018, 19, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Maxon, H.R.; Thomas, S.R.; Hertzberg, V.S.; Kereiakes, J.G.; Chen, I.-W.; Sperling, M.I.; Saenger, E.L. Relation between effective radiation dose and outcome of radioiodine therapy of thyroid cancer. N. Engl. J. Med. 1983, 309, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Maxon, H.R.; Smith, H.S. Radioiodine-131 in the diagnosis and treatment of metastatic well differentiated thyroid cancer. Endocrinol. Metab. Clin. N. Am. 1990, 19, 685–718. [Google Scholar] [CrossRef]
- Stabin, M.G.; Sparks, R.B.; Crowe, E. OLINDA/EXM: The second-generation personal computer software for internal dose assessment in nuclear medicine. J. Nucl. Med. 2005, 46, 1023–1027. [Google Scholar] [CrossRef]
- Hänscheid, H.; Lassmann, M.; Luster, M.; Kloos, R.T.; Reiners, C. Blood dosimetry from a single measurement of the whole body radioiodine retention in patients with differentiated thyroid carcinoma. Endocr. Relat. Cancer 2009, 16, 1283–1289. [Google Scholar] [CrossRef]
- Thomas, S.R.; Samaratunga, R.S.; Sperling, M.; Maxon, H.R., 3rd. Predictive estimate of blood dose from external counting data preceding radioiodine therapy for thyroid cancer. Nucl. Med. Biol. 1993, 20, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Sisson, J.C.; Shulkin, B.L.; Lawson, B.S. Increasing efficacy and safety of treatments of patients with well-differentiated thyroid carcinoma by measuring body retentions of 131I. J. Nucl. Med. 2003, 44, 898–903. [Google Scholar] [PubMed]
- Kuker, R.; Georgiou, M.; Gomez-Marin, O.; Serafini, A.; Sfakianakis, G. Feasibility of an abbreviated protocol for dosimetry-guided I-131 therapy. J. Nucl. Med. 2007, 48 (Suppl. 2), 17P. [Google Scholar]
- Mahajan, P.S. Artificial Intelligence in Healthcare: AI, Machine Learning, and Deep and Intelligent Medicine Simplified for Everyone; MedMantra, LLC: Albuquerque, NM, USA, 2022. [Google Scholar]
- Saboury, B.; Bradshaw, T.; Boellaard, R.; Buvat, I.; Dutta, J.; Hatt, M.; Jha, A.K.; Li, Q.; Liu, C.; McMeekin, H. Artificial Intelligence in Nuclear Medicine: Opportunities, challenges and responsibilities toward a trustworthy ecosystem. J. Nucl. Med. 2023, 64, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, T.J.; Boellaard, R.; Dutta, J.; Jha, K.A.; Jacobs, P.; Li, Q.; Liu, C.; Sitek, A.; Saboury, B.; Scott, J.H.P.; et al. Nuclear Medicine and Artificial Intelligence: Best Practices for Algorithm Development. J. Nucl. Med. 2022, 63, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Jain, A.; Araveeti, S.R.; Adhikari, S.; Garg, H.; Bhandari, M. FDA approved Artificial Intelligence and Machine Learning (AI/ML)-enabled medical devices: An updated landscape. medRxiv 2022. [Google Scholar] [CrossRef]
- Jha, A.K.; Bradshaw, T.J.; Buvat, I.; Hatt, M.; Kc, P.; Liu, C.; Obuchowski, N.F.; Saboury, B.; Slomka, P.J.; Sunderland, J.J.; et al. Nuclear Medicine and Artificial Intelligence: Best practices for evaluation (the RELAINCE guidelines). J. Nucl. Med. 2022, 63, 1288–1299. [Google Scholar] [CrossRef]
- Zukotynski, K.; Gaudet, V.; Uribe, C.F.; Mathotaarachchi Smith, K.C.; Rosa-Neto, P.; Benard, F.; Black, S.E. Machine Learning in Nuclear Medicine: Part 2—Neural Networks and Clinical Aspects. J. Nucl. Med. 2021, 62, 22–29. [Google Scholar] [CrossRef]



| No. Sex, Age, y | All | Male | Female |
|---|---|---|---|
| Patients (n) | 83 | 30 | 53 |
| Mean Age (y) | 48.2 | 45.9 | 48.8 |
| Mean Weight (lbs) | 161.1 | 181.4 | 149.6 |
| Average Calculated Maximum Permissible Activity (mCi) | 456 (range 117–1080) |
| Patient # | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIRD (mCi) | 140 | 791 | 230 | 310 | 722 | 625 | 529 | 770 | 342 | 762 | 525 | 804 | 615 |
| AI (mCi) | 129 | 778 | 219 | 275 | 756 | 622 | 551 | 754 | 341 | 701 | 534 | 798 | 624 |
| Difference (mCi) | −11 | −14 | −10 | −35 | 34 | −3 | 22 | −16 | −1 | −61 | 9 | −6 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiou, M.F.; Nielsen, J.A.; Chiriboga, R.; Kuker, R.A. An Artificial Intelligence System for Optimizing Radioactive Iodine Therapy Dosimetry. J. Clin. Med. 2024, 13, 117. https://doi.org/10.3390/jcm13010117
Georgiou MF, Nielsen JA, Chiriboga R, Kuker RA. An Artificial Intelligence System for Optimizing Radioactive Iodine Therapy Dosimetry. Journal of Clinical Medicine. 2024; 13(1):117. https://doi.org/10.3390/jcm13010117
Chicago/Turabian StyleGeorgiou, Michalis F., Joshua A. Nielsen, Rommel Chiriboga, and Russ A. Kuker. 2024. "An Artificial Intelligence System for Optimizing Radioactive Iodine Therapy Dosimetry" Journal of Clinical Medicine 13, no. 1: 117. https://doi.org/10.3390/jcm13010117
APA StyleGeorgiou, M. F., Nielsen, J. A., Chiriboga, R., & Kuker, R. A. (2024). An Artificial Intelligence System for Optimizing Radioactive Iodine Therapy Dosimetry. Journal of Clinical Medicine, 13(1), 117. https://doi.org/10.3390/jcm13010117