Oxidative Stress Mediates Epigenetic Modifications and the Expression of miRNAs and Genes Related to Apoptosis in Diabetic Retinopathy Patients
Abstract
:1. Introduction
2. Methods
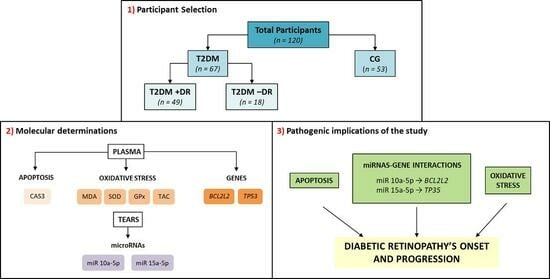
2.1. Study Design and Participant Characteristics
2.2. Proceedings
2.2.1. Demographic Characteristics of the Study Participants
2.2.2. Clinical Characteristics of the Study Participants
2.2.3. Sample Proceedings
2.2.4. Biochemical and Molecular-Genetic Variables of the Study Participants
Clinical Biochemistry
Oxidative Stress
Apoptosis
MicroRNAs
Gene Expression
2.2.5. General Statistics and Bioinformatics
3. Results
3.1. Demographics and Participant Characteristics
3.2. Ophthalmologic Evaluation
3.3. Biochemical Variables
3.4. Molecular-Genetic Variables
3.4.1. Oxidative Stress
3.4.2. Apoptosis
3.4.3. miRNAs
3.4.4. Gene Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMA | American Medical Association |
| AP | apoptosis |
| Bad | Bcl-2 antagonist of cell death |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | intrinsic mitochondrial B-cell lymphoma 2 (Bcl-2) family proteins |
| Bcl-XL | B-cell lymphoma-extra large |
| BCL2L2 | B2 cell lymphoma gene |
| BCVA | best corrected visual acuity |
| BH3-only | proapoptotic homology (BH)3-only |
| Bmf | Bcl2 modifying factor |
| BMGB | biochemical and molecular-genetic biomarkers |
| BMC | biomicroscopy |
| c-FLIP | cellular FLICE, FADD-like IL-1β-converting enzyme-inhibitory protein |
| CAS3 | caspase-3 |
| CAT | catalase |
| CG | control group |
| CREAT | creatinine |
| DISC | death-inducing signaling complex |
| DME | diabetic macular edema |
| ELISA | enzyme-linked immunosorbent assays |
| FasL | Fas ligand |
| FADD | Fas-associated death domain |
| GPx | glutathione peroxidase |
| GPx 4 | glutathione peroxidase 4 gene |
| H2O2 | hydrogen peroxide |
| HbA1c | glycated hemoglobin |
| HDL | high-density lipoprotein cholesterol |
| LDL | low-density lipoprotein cholesterol |
| LE | left eye |
| LogMAR | logarithm of the minimum angle of resolution |
| mRNA | messenger ribonucleic acid |
| miR/miRNA | microribonucleic acid |
| MDA | malondialdehyde |
| NGF | nerve growth factor |
| NF-κB | nuclear factor kappa B |
| NOXA | phorbol-12-myristate-13-acetate-induced protein |
| NPDR | nonproliferative diabetic retinopathy |
| Nrf2 | nuclear factor (erythroid-derived 2)-like 2 |
| NVU | neurovascular unit |
| OCT | optical coherence tomography |
| OCTA | optical coherence tomography angiography |
| OF | ocular fundus |
| OH | hydroxyl radical |
| OS | oxidative stress |
| PDR | proliferative diabetic retinopathy |
| PUMA | p53 upregulated modulator of apoptosis |
| RE | right eye |
| RNFL | retinal nerve fiber layer |
| ROS | reactive oxygen species |
| RTG | retinography |
| SD | standard deviation |
| SOD | superoxide dismutase |
| TAC | total antioxidant capacity |
| TBARS | thiobarbituric acid reactive substances |
| TNF-α | tumor necrosis factor alpha |
| TRADD | TNFR1-associated death domain protein |
| TRIG | triglycerides |
| Total Chol | total cholesterol |
| TP53 | tumor protein p53 gene |
| TWEAK | TNF-like weak inducer of apoptosis |
| TRAIL | tumor necrosis factor (TNF)-related apoptosis-inducing ligand |
| VEGF | vascular endothelial growth factor |
| VLDL | very low-density lipoprotein cholesterol |
References
- Vujosevic, S.; Aldington, S.J.; Silva, P.; Hernández, C.; Scanlon, P.; Peto, T.; Simó, R. Screening for diabetic retinopathy: New perspectives and challenges. Lancet Diabetes Endocrinol. 2020, 8, 337–347. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Cheng, C.Y. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Amoaku, W.M.; Ghanchi, F.; Bailey, C.; Banerjee, S.; Banerjee, S.; Downey, L.; Gale, R.; Hamilton, R.; Khunti, K.; Posner, E.; et al. Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group. Eye 2020, 34, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Haydinger, C.D.; Oliver, G.F.; Ashander, L.M.; Smith, J.R. Oxidative Stress and Its Regulation in Diabetic Retinopathy. Antioxidants 2023, 12, 1649. [Google Scholar] [CrossRef] [PubMed]
- Nian, S.; Lo, A.C.Y.; Mi, Y.; Ren, K.; Yang, D. Neurovascular unit in diabetic retinopathy: Pathophysiological roles and potential therapeutical targets. Eye Vis. 2021, 8, 15. [Google Scholar] [CrossRef]
- Lin, K.Y.; Hsih, W.H.; Lin, Y.B.; Wen, C.Y.; Chang, T.J. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J. Diabetes Investig. 2021, 12, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Posarelli, C.; Sartini, F.; Casini, G.; Passani, A.; Toro, M.D.; Vella, G.; Figus, M. What Is the Impact of Intraoperative Micro-scope-Integrated OCT in Ophthalmic Surgery? Relevant Applications and Outcomes. A Systematic Review. J. Clin. Med. 2020, 9, 1682. [Google Scholar]
- Mehta, N.; Tsui, E.; Lee, G.D.; Dedania, V.; Modi, Y. Imaging Biomarkers in Diabetic Retinopathy and Diabetic Macular Edema. Int. Ophthalmol. Clin. 2019, 59, 241–262. [Google Scholar] [CrossRef]
- Nanegrungsunk, O.; Patikulsila, D.; Sadda, S.R. Ophthalmic imaging in diabetic retinopathy: A review. Clin. Exp. Ophthalmol. 2022, 50, 1082–1096. [Google Scholar] [CrossRef]
- Owsley, C.; McGwin, G., Jr.; Lee, D.J.; Lam, B.L.; Friedman, D.S.; Gower, E.W.; Haller, J.A.; Hark, L.A.; Saladdine, J. Innovative Network for Sight (INSIGHT) Research Group. Diabetes eye screening in urban settings serving minority populations: Detection of diabetic retinopathy and other ocular findings using telemedicine. JAMA Ophthalmol. 2015, 133, 174–181. [Google Scholar] [CrossRef]
- Grzybowski, A.; Brona, P.; Lim, G.; Ruamviboonsuk, P.; Tan, G.S.W.; Abramoff, M.; Ting, D.S.W. Artificial intelligence for diabetic retinopathy screening: A review. Eye 2020, 34, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Gurung, R.L.; FitzGerald, L.M.; McComish, B.J.; Verma, N.; Burdon, K.P. Identifying Genetic Risk Factors for Diabetic Macular Edema and the Response to Treatment. J. Diabetes Res. 2020, 2020, 5016916. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Blasco, I.; Gallego-Martínez, A.; Machado, X.; Cruz-Espinosa, J.; Di Lauro, S.; Casaroli-Marano, R.; Alegre-Ituarte, V.; Arévalo, J.F.; Pinazo-Durán, M.D. Oxidative Stress, Inflammatory, Angiogenic, and Apoptotic molecules in Proliferative Diabetic Retinopathy and Diabetic Macular Edema Patients. Int. J. Mol. Sci. 2023, 24, 8227. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, S.; Pan, Y.; Jin, M.; Li, J.; Luo, Y.; Sun, X.; Li, G. Diabetic retinopathy: Involved cells, biomarkers, and treatments. Front. Pharmacol. 2022, 13, 953691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Zhang, C.; Zhang, J.; Gu, L.; Luo, D.; Qiu, Q. Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells 2022, 11, 3362. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tan, T.-E.; Shao, Y.; Wong, T.Y.; Li, X. Classification of diabetic retinopathy: Past, present and future. Front. Endocrinol. 2022, 13, 1079217. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.A.; Rao, V.R.; Chen, S.; Cao, D.; Gao, X.; Cleary, P.A.; Huang, R.S.; Paterson, A.D.; Natarajan, R.; Rehman, J.; et al. Lymphoblastoid Cell Lines as a Tool to Study Inter-Individual Differences in the Response to Glucose. PLoS ONE 2016, 11, e0160504. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Pinazo-Durán, M.; Gallego-Pinazo, R.; Medina, J.J.G.; Zanón-Moreno, V.; Nucci, C.; Dolz-Marco, R.; Martinez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramírez, C.; López-Gálvez, M.I.; et al. Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging 2014, 9, 637–652. [Google Scholar] [CrossRef]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 147–171. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Aliaga, I.; Thor, S. Programmed cell death in the nervous system-a programmed cell fate? Curr. Opin. Neurobiol. 2009, 2, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Turk, B. Molecular mechanisms of cell death: Recommen-dations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Bedoui, S.; Herold, M.J.; Strasser, A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 2020, 21, 678–695. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.-E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Shin, S.; Jung, Y.; Uhm, H.; Song, M.; Son, S.; Goo, J.; Jeong, C.; Song, J.-J.; Kim, V.N.; Hohng, S. Quantification of purified endogenous miRNAs with high sensitivity and specificity. Nat. Commun. 2020, 11, 6033. [Google Scholar] [CrossRef]
- Sahajpal, N.S.; Goel, R.K.; Chaubey, A.; Aurora, R.; Jain, S.K. Pathological Perturbations in Diabetic Retinopathy: Hypergly-cemia, AGEs, Oxidative Stress and Inflammatory Pathways. Curr. Protein Pept. Sci. 2019, 20, 92–110. [Google Scholar] [CrossRef]
- Satari, M.; Aghadavod, E.; Mobini, M.; Asemi, Z. Association between miRNAs expression and signaling pathways of oxidative stress in diabetic retinopathy. J. Cell. Physiol. 2018, 234, 8522–8532. [Google Scholar] [CrossRef]
- Fioravanti, A.; Giordano, A.; Dotta, F.; Pirtoli, L. Crosstalk between MicroRNA and Oxidative Stress in Physiology and Pa-thology 2. Int. J. Mol. Sci. 2022, 23, 6831. [Google Scholar] [CrossRef]
- Wang, H.D.; Allard, P. Challenging dogmas: How transgenerational epigenetics reshapes our views on life. J. Exp. Zool. Part A: Ecol. Integr. Physiol. 2021, 337, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Ricke, D.O.; Ng, D.; Michaleas, A.; Fremont-Smith, P. Omics Analysis and Quality Control Pipelines in a High-Performance Computing Environment. OMICS A J. Integr. Biol. 2023, 27, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Arif, K.M.T.; Elliott, E.K.; Haupt, L.M.; Griffiths, L.R. Regulatory Mechanisms of Epigenetic miRNA Relationships in Human Cancer and Potential as Therapeutic Targets. Cancers 2020, 12, 2922. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, F.; D’aversa, E.; Serino, M.L.; Singh, A.V.; Secchiero, P.; Zauli, G.; Tisato, V.; Gemmati, D. miRNAs Epigenetic Tuning of Wall Remodeling in the Early Phase after Myocardial Infarction: A Novel Epidrug Approach. Int. J. Mol. Sci. 2023, 24, 13268. [Google Scholar] [CrossRef] [PubMed]
- Hossan, T.; Kundu, S.; Alam, S.S.; Nagarajan, S. Epigenetic Modifications Associated with the Pathogenesis of Type 2 Diabetes Mellitus. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 775–786. [Google Scholar] [CrossRef]
- Pinazo-Durán, M.D.; Galbis-Estrada, C.; Pons-Vázquez, S.; Cantú-Dibildox, J.; Marco-Ramírez, C.; Benítez-del-Castillo, J. Effects of a nutraceutical formulation based on the combination of antioxidants and ω-3 essential fatty acids in the expression of in-flammation and immune response mediators in tears from patients with dry eye disorders. Clin. Interv. Aging 2013, 8, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Del-Castillo, J.; Cantu-Dibildox, J.; Sanz-González, S.M.; Zanón-Moreno, V.; Pinazo-Duran, M.D. Cytokine expression in tears of patients with glaucoma or dry eye disease: A prospective, observational cohort study. Eur. J. Ophthalmol. 2018, 29, 437–443. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Ateş, N.; Yildirim, Ö.; Tamer, L.; Ünlü, A.; Ercan, B.; Muşlu, N.; Kanik, A.; Hatungil, R.; Atik, U. Plasma catalase activity and malondialdehyde level in patients with cataract. Eye 2004, 18, 785–788. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2009, 5, 51–66. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Costa, J.O.; Vásquez, C.M.P.; Santana, G.D.J.; Silva, N.D.J.; Braz, J.D.M.; De Jesus, A.M.R.; Da Silva, D.G.; Cunha, L.C.S.; Barbosa, K.B.F. Plasma Total Antioxidant Capacity and Cardiometabolic Risk in Non-Obese and Clinically Healthy Young Adults. Arq. Bras. Cardiol. 2017, 109, 140–147. [Google Scholar] [CrossRef]
- Pyrshev, K.A.; Yesylevskyy, S.O.; Mély, Y.; Demchenko, A.P.; Klymchenko, A.S. Caspase-3 activation decreases lipid order in the outer plasma membrane leaflet during apoptosis: A fluorescent probe study. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2123–2132. [Google Scholar] [CrossRef]
- Song, J.; Jiao, Y.; Zhang, T.; Zhang, Y.; Huang, X.; Li, H.; Wu, H. Longitudinal Changes in Plasma Caspase-1 and Caspase-3 during the First 2 Years of HIV-1 Infection in CD4Low and CD4High Patient Groups. PLoS ONE 2015, 10, e0121011. [Google Scholar] [CrossRef]
- Raga-Cervera, J.; Bolarin, J.M.; Millan, J.M.; Garcia-Medina, J.J.; Pedrola, L.; Abellán-Abenza, J.; Valero-Vello, M.; Sanz-González, S.M.; O’connor, J.E.; Galarreta-Mira, D.; et al. miRNAs and Genes Involved in the Interplay between Ocular Hypertension and Primary Open-Angle Glaucoma. Oxidative Stress, Inflammation, and Apoptosis Networks. J. Clin. Med. 2021, 10, 2227. [Google Scholar] [CrossRef]
- Altman, J.; Jones, G.; Ahmed, S.; Sharma, S.; Sharma, A. Tear Film MicroRNAs as Potential Biomarkers: A Review. Int. J. Mol. Sci. 2023, 24, 3694. [Google Scholar] [CrossRef]
- Peng, X.; Xianbin, L.; Yijun, L.; Zhenshen, B.; Fengyue, Z.; Lili, G.; Saeed, K.; Wenbin, L. PmiRtarbase: A positive miR-NA-target regulations database. Comput. Biol. Chem. 2022, 98, 107690. [Google Scholar]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA Using TRIzol (TRI Reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb-prot5439. [Google Scholar] [CrossRef]
- Hieronymus, K.; Dorschner, B.; Schulze, F.; Vora, N.L.; Parker, J.S.; Winkler, J.L.; Rösen-Wolff, A.; Winkler, S. Validation of reference genes for whole blood gene expression analysis in cord blood of preterm and full-term neonates and peripheral blood of healthy adults. BMC Genom. 2021, 22, 489. [Google Scholar] [CrossRef]
- Zeller, T.; Blankerberg, S. Blood-Based Gene Expression Tests. Promises and Limitations. Circ. Cardiovasc. Gen. 2013, 6, 139–140. [Google Scholar] [CrossRef]
- Li, Z.; Tong, J.; Liu, C.; Zhu, M.; Tan, J.; Kuang, G. Analysis of independent risk factors for progression of different degrees of diabetic retinopathy as well as non-diabetic retinopathy among type 2 diabetic patients. Front. Neurosci. 2023, 17, 1143476. [Google Scholar] [CrossRef]
- Bjornstad, P.; Domalpally, A.; Drews, K.L.; Gubitosi-Klug, R.; Levitsky, L.L.; Pak, J.W.; White, N.H.; Blodi, B.A. Retinal Thickness and Morphology Changes on OCT in Youth with Type 2 Diabetes: Findings from the TODAY Study. Ophthalmol. Sci. 2022, 2, 100191. [Google Scholar]
- Liang, J.; Lei, W.; Cheng, J. Correlations of blood lipids with early changes in macular thickness in patients with diabetes. J. Français Ophtalmol. 2019, 42, 276–280. [Google Scholar] [CrossRef]
- Sasaki, M.; Kawashima, M.; Kawasaki, R.; Uchida, A.; Koto, T.; Shinoda, H.; Tsubota, K.; Wang, J.J.; Ozawa, Y. Association of Serum Lipids with Macular Thickness and Volume in Type 2 Diabetes Without Diabetic Macular Edema. Investig. Opthalmol. Vis. Sci. 2014, 55, 1749–1753. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Todkar, K.; Ilamathi, H.S.; Germain, M. Mitochondria and Lysosomes: Discovering Bonds. Front. Cell Dev. Biol. 2017, 5, 106. [Google Scholar] [CrossRef]
- Demers-Lamarche, J.; Guillebaud, G.; Tlili, M.; Todkar, K.; Bélanger, N.; Grondin, M.; Nguyen, A.P.; Michel, J.; Germain, M. Loss of Mitochondrial Function Impairs Lysosomes. J. Biol. Chem. 2016, 291, 10263–10276. [Google Scholar] [CrossRef]
- Hempel, N.; Melendez, J.A. Intracellular redox status controls membrane localization of pro- and anti-migratory signaling molecules. Redox Biol. 2014, 2, 245–250. [Google Scholar] [CrossRef]
- Doly, M.; Droy-Lefaix, M.T.; Braquet, P. Oxidative stress in diabetic retina. EXS 1992, 62, 299–307. [Google Scholar]
- Sanz-González, S.M.; García-Medina, J.J.; Zanón-Moreno, V.; López-Gálvez, M.I.; Galarreta-Mira, D.; Duarte, L.; Valero-Velló, M.; Ramírez, A.I.; Arévalo, J.F.; Pinazo-Durán, M.D. Clinical and Molecular-Genetic Insights into the Role of Oxidative Stress in Diabetic Retinopathy: Antioxidant Strategies and Future Avenues. Antioxidants 2020, 9, 1101. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Foulquie-Moreno, E.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D.; Zanon-Moreno, V.; Del-Rio-Vellosillo, M. Update on the Effects of Antioxidants on Diabetic Retinopathy: In Vitro Experiments, Animal Studies and Clinical Trials. Antioxidants 2020, 9, 561. [Google Scholar] [CrossRef]
- Roig-Revert, M.J.; Lleó-Pérez, A.; Zanón-Moreno, V.; Vivar-Llopis, B.; Marín-Montiel, J.; Dolz-Marco, R.; Alonso-Muñoz, L.; Albert-Fort, M.; López-Gálvez, M.I.; Galarreta-Mira, D.; et al. Valencia Study on Diabetic Retinopathy (VSDR). Enhanced Oxidative Stress and Other Potential Biomarkers for Retinopathy in Type 2 Diabetics: Beneficial Effects of the Nutraceutic Supplements. Biomed. Res. Int. 2015, 2015, 408180. [Google Scholar] [CrossRef]
- Madsen-Bouterse, S.A.; Kowluru, R.A. Oxidative stress and diabetic retinopathy: Pathophysiological mechanisms and treat-ment perspectives. Rev. Endocr. Metab. Disord. 2008, 9, 315–327. [Google Scholar] [CrossRef]
- Carnevale, C.; Domanico, D.; Fragiotta, S.; Cutini, A.; Zompatori, L.; Vingolo, E. Circulating levels of reactive oxygen species in patients with nonproliferative diabetic retinopathy and the influence of antioxidant supplementation: 6-month follow-up. Indian J. Ophthalmol. 2015, 63, 9–14. [Google Scholar] [CrossRef]
- Pan, J.; Liu, S.; Farkas, M.; Consugar, M.; Zack, D.J.; Kozak, I. Serum molecular signature for proliferative diabetic retinopathy in Saudi patients with type 2 diabetes. Mol. Vis. 2016, 22, 636–645. [Google Scholar]
- Khan, A.A.; Rahmani, A.H.; Aldebasi, Y.H. Diabetic Retinopathy: Recent Updates on Different Biomarkers and Some Therapeutic Agents. Curr. Diabetes Rev. 2018, 14, 523–533. [Google Scholar] [CrossRef]
- Wu, T.; Qiao, S.; Shi, C.; Wang, S.; Ji, G. Metabolomics window into diabetic complications. J. Diabetes Investig. 2017, 9, 244–255. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, H.; Guan, W.; Kang, X.; Tai, X.; Shen, Y. Metabolic memory in mitochondrial oxidative damage triggers diabetic retinopathy. BMC Ophthalmol. 2018, 18, 258. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, X.; Hu, Y.; Li, T.; Gao, Y.; Shi, Y.; Tang, S. Mitochondrial DNA oxidative damage triggering mitochondrial dys-function and apoptosis in high glucose-induced HRECs. Invest. Ophthalmol. Vis. Sci. 2008, 49, 4203–4209. [Google Scholar]
- Cuadrado, A.; Martín-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription factors NRF2 and NF-κB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J. Biol. Chem. 2014, 289, 15244–15258. [Google Scholar] [CrossRef]
- Jänicke, R.U.; Sprengart, M.L.; Wati, M.R.; Porter, A.G. Caspase-3 Is Required for DNA Fragmentation and Morphological Changes Associated with Apoptosis. J. Biol. Chem. 1998, 273, 9357–9360. [Google Scholar] [CrossRef]
- Valverde, A.M.; Miranda, S.; García-Ramírez, M.; González-Rodriguez, Á.; Hernández, C.; Simó, R. Proapoptotic and survival signaling in the neuroretina at early stages of diabetic retinopathy. Mol. Vis. 2013, 19, 47–53. [Google Scholar]
- Kowluru, R.A. Cross Talks between Oxidative Stress, Inflammation and Epigenetics in Diabetic Retinopathy. Cells 2023, 12, 300. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Hossein-Nezhad, A.; Larijani, B.; Amini, M.; Keshtkar, A. Global DNA methylation as a possible biomarker for diabetic retinopathy. Diabetes Metab. Res. Rev. 2014, 31, 183–189. [Google Scholar] [CrossRef]
- Chao, Y.; Gu, T.; Zhang, Z.; Wu, T.; Wang, J.; Bi, Y. The role of miRNAs carried by extracellular vesicles in type 2 diabetes and its complications. J. Diabetes 2023, 15, 838–852. [Google Scholar] [CrossRef]
- Vu, T.T.; Stölzel, F.; Wang, K.W.; Röllig, C.; Tursky, M.L.; Molloy, T.J.; Ma, D.D. miR-10a as a therapeutic target and predictive biomarker for MDM2 inhibition in acute myeloid leukemia. Leukemia 2020, 35, 1933–1948. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Jamal, H.H.; Taheri, M.; Hajiesmaeili, M. A Comprehensive Review on Function of miR-15b-5p in Malignant and Non-Malignant Disorders. Front. Oncol. 2022, 12, 870996. [Google Scholar] [CrossRef]
- Bhatlekar, S.; Basak, I.; Edelstein, L.C.; Campbell, R.A.; Lindsey, C.R.; Italiano, J.E., Jr.; Weyrich, A.S.; Rowley, J.W.; Rondina, M.T.; Sola-Visner, M.; et al. Anti-apoptotic BCL2L2 increases megakaryocyte proplatelet formation in cultures of human cord blood. Haematologica 2019, 104, 2075–2083. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures, and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef]
- Parker, E.D.; Lin, J.; Mahoney, T.; Ume, N.; Yang, G.; Gabbay, R.A.; ElSayed, N.A.; Bannuru, R.R. Economic Costs of Diabetes in the U.S. in 2022. Diabetes Care 2023, 1, dci230085. [Google Scholar] [CrossRef] [PubMed]






| INCLUSION | EXCLUSION |
|---|---|
|
|
| Variables | CG | T2DM−DR | T2DM+DR | p-Value (χ2) |
|---|---|---|---|---|
| HBP (%) | - | 44 (62.9%) | 44 (75.9%) | 4.92 × 10−18 * |
| FH of T2DM (%) | 14 (23.3%) | 16 (22.9%) | 28 (48.3%) | 5.35 × 10−19 * |
| FH of HBP (%) | 28 (46.7%) | 16 (22.9%) | 34 (58.6%) | 1.63 × 10−16 * |
| Alcohol intake (%) | 52 (86.6%) | 42 (60%) | 28 (31%) | 2.21 × 10−7 * |
| Tobacco use (%) | 30 (50%) | 28 (40%) | 10 (17.2%) | 0.000055 * |
| BMI (kg/m2) | 23.57 ± 3.28 | 26.72 ± 3.54 | 26.84 ± 2.80 | 1.5091 × 10−8 * |
| Physical exercise (%) | 42 (70%) | 32 (55.7%) | 4 (6.9%) | 2.91 × 10−12 * |
| Variables | CG | T2DM−DR | T2DM+DR | p-Value (ANOVA) |
|---|---|---|---|---|
| BCVA (LogMAR) | 0.037 ± 0.09 | 0.042 ± 0.08 | 0.127 ± 0.22 | 0.014 * |
| IOP (mmHg) | 15.7 ± 2.4 | 15 ± 2 | 14.9 ± 1.9 | 0.912 |
| CST (mm) | 258.26 ± 31.23 | 252.51 ± 20.39 | 285.1 ± 45.17 | 3.86 × 10−8 * |
| CV (mm3) | 0.205 ± 0.019 | 0.208 ± 0.06 | 0.235 ± 0.013 | 1.25 × 10−28 * |
| Variables | CG | T2DM−DR | T2DM+DR | p-Value |
|---|---|---|---|---|
| Glycemia (mg/dL) | 91 ± 9 | 98 ± 14 | 97 ± 13 | 0.011 * |
| HbA1c (%) | 5.64 ± 0.37 | 7.07 ± 0.41 | 7.25 ± 0.66 | 3.63 × 10−46 * |
| T Chol (mg/dL) | 170 ± 40 | 159 ± 22 | 172 ± 22 | 0.029 * |
| VLDL (mg/dL) | 18 ± 5 | 32 ± 5 | 42 ± 9 | 8.87 × 10−49 * |
| LDL (mg/dL) | 95 ± 31 | 103 ± 23 | 112 ± 19 | 0.002 * |
| HDL (mg/dL) | 59 ± 13 | 53 ± 8 | 55 ± 10 | 0.008 * |
| TRIG (mg/dL) | 83 ± 30 | 91 ± 9 | 91 ± 13 | 0.042 * |
| Urea (mg/dL) | 35 ± 8 | 38 ± 11 | 38 ± 11 | 0.391 |
| CREAT (mg/dL) | 0.93 ± 0.18 | 0.93 ± 0.21 | 0.94 ± 0.25 | 0.992 |
| Iron (mg/dL) | 76 ± 22 | 89 ± 31 | 77 ± 23 | 0.125 |
| CST (mm) | CV (mm3) | |||
|---|---|---|---|---|
| Biochemical Data | PCC | PPC p-Value | PCC | PPC p-Value |
| Basal glycemia | −0.026 | 0.726 | 0.050 | 0.493 |
| HbA1c | 0.144 * | 0.049 | 0.275 ** | <0.001 |
| T Chol | 0.110 | 0.132 | 0.262 ** | <0.001 |
| VLDL | 0.277 ** | <0.001 | 0.443 ** | <0.001 |
| LDL | 0.189 ** | 0.009 | 0.352 ** | <0.001 |
| HDL | −0.044 | 0.552 | −0.046 | 0.535 |
| TRIG | 0.094 | 0.197 | 0.085 | 0.248 |
| BMI | −0.046 | 0.531 | 0.123 | 0.094 |
| T2DM vs. CG | T2DM+DR vs. T2DM−DR | ||||||
|---|---|---|---|---|---|---|---|
| Upregulated | p-Value | Downregulated | p-Value | Upregulated | p-Value | Downregulated | p-Value |
| hsa-miR-155-5p | 0.00050289 | hsa-miR-10a-5p | 0.00021554 | hsa-miR-147b | 0.00033305 | hsa-miR-342-3p | 0.0006826 |
| hsa-miR-4488 | 0.00215255 | hsa-miR-195-3p | 0.00344617 | hsa-miR-31-5p | 0.00356039 | hsa-miR-148a-3p | 0.00137003 |
| hsa-miR-4516 | 0.00267474 | hsa-miR-135a-5p | 0.01745868 | hsa-miR-34a-5p | 0.00550053 | hsa-miR-27a-5p | 0.00463293 |
| hsa-miR-92b-5p | 0.00352394 | hsa-miR-320a | 0.01795334 | hsa-miR-4436b-3p | 0.00667778 | hsa-miR-423-5p | 0.02206753 |
| hsa-miR-15b-5p | 0.01224091 | hsa-miR-342-5p | 0.01846176 | hsa-miR-3158-3p | 0.0076641 | hsa-miR-9-3p | 0.02287876 |
| hsa-miR-139-5p | 0.01696883 | hsa-miR-486-5p | 0.02715084 | hsa-miR-508-3p | 0.00797464 | hsa-miR-195-3p | 0.02605848 |
| hsa-miR-203 | 0.04464176 | hsa-miR-155-5p | 0.01040889 | hsa-miR-4794 | 0.02879332 | ||
| hsa-miR-378a-3p | 0.04503972 | hsa-miR-450b-5p | 0.01488504 | hsa-miR-493-3p | 0.02879332 | ||
| T2DM−DR vs. CG | hsa-miR-20b-5p | 0.01673733 | hsa-miR-550a-3p | 0.02879332 | |||
| Upregulated | p-value | Downregulated | p-value | hsa-miR-211-5p | 0.01899113 | hsa-miR-204-3p | 0.03643016 |
| hsa-miR-155-5p | 0.00048824 | hsa-miR-10a-5p | 0.00022013 | hsa-miR-1287 | 0.02255178 | hsa-miR-3648 | 0.03705494 |
| hsa-miR-15b-5p | 0.00405801 | hsa-miR-452-5p | 0.00069978 | hsa-miR-203 | 0.02305019 | hsa-miR-625-5p | 0.03804594 |
| hsa-miR-375 | 0.01056473 | hsa-miR-186-5p | 0.00282549 | hsa-miR-504 | 0.02427954 | hsa-miR-4638-3p | 0.04095184 |
| hsa-miR-708-3p | 0.01085626 | hsa-miR-34a-5p | 0.01578009 | hsa-miR-455-5p | 0.02587983 | hsa-miR-451a | 0.04272808 |
| hsa-miR-1260a | 0.01168696 | hsa-miR-324-3p | 0.01956076 | hsa-miR-505-3p | 0.02702551 | ||
| hsa-miR-184 | 0.02463651 | hsa-miR-195-3p | 0.02066843 | hsa-miR-30c-2-3p | 0.02736074 | ||
| hsa-miR-92b-5p | 0.03447955 | hsa-miR-27a-5p | 0.02106449 | hsa-miR-550a-3-5p | 0.0277247 | ||
| hsa-miR-103a-3p | 0.02243327 | hsa-miR-15b-5p | 0.03226176 | ||||
| hsa-miR-30e-5p | 0.02258532 | hsa-miR-651 | 0.03254246 | ||||
| hsa-miR-29b-2-5p | 0.02387301 | hsa-miR-720 | 0.03528564 | ||||
| hsa-miR-342-5p | 0.02391494 | hsa-miR-675-3p | 0.03571653 | ||||
| hsa-miR-193b-5p | 0.02683451 | hsa-miR-4662a-5p | 0.0365617 | ||||
| T2DM+DR vs. CG | hsa-miR-942 | 0.03822863 | |||||
| Upregulated | p-value | Downregulated | p-value | hsa-miR-330-5p | 0.03836754 | ||
| hsa-miR-15b-5p | 0.00038556 | hsa-miR-10a-5p | 0.00010335 | hsa-miR-1278 | 0.03912887 | ||
| hsa-miR-155-5p | 0.00041997 | hsa-miR-195-3p | 0.00107758 | hsa-miR-30b-3p | 0.04009894 | ||
| hsa-miR-342-3p | 0.00233141 | hsa-miR-451a | 0.00651537 | hsa-miR-4446-3p | 0.04031481 | ||
| hsa-miR-27a-5p | 0.00360745 | hsa-miR-203 | 0.01323233 | hsa-miR-19a-3p | 0.04039565 | ||
| hsa-miR-423-3p | 0.01721141 | hsa-miR-211-5p | 0.02409484 | hsa-miR-130b-5p | 0.04109527 | ||
| hsa-miR-328 | 0.0327679 | hsa-let-7a-3p | 0.02572669 | hsa-miR-92b-5p | 0.04321649 | ||
| hsa-miR-375 | 0.04351622 | hsa-miR-27b-5p | 0.0448958 | ||||
| hsa-miR-184 | 0.04767438 | hsa-miR-3126-5p | 0.04586702 | ||||
| hsa-miR-204-3p | 0.04781022 | hsa-miR-501-3p | 0.04778805 | ||||
| hsa-miR-324-3p | 0.04801066 | ||||||
| hsa-miR-708-3p | 0.04923371 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karam-Palos, S.; Andrés-Blasco, I.; Campos-Borges, C.; Zanón-Moreno, V.; Gallego-Martínez, A.; Alegre-Ituarte, V.; García-Medina, J.J.; Pastor-Idoate, S.; Sellés-Navarro, I.; Vila-Arteaga, J.; et al. Oxidative Stress Mediates Epigenetic Modifications and the Expression of miRNAs and Genes Related to Apoptosis in Diabetic Retinopathy Patients. J. Clin. Med. 2024, 13, 74. https://doi.org/10.3390/jcm13010074
Karam-Palos S, Andrés-Blasco I, Campos-Borges C, Zanón-Moreno V, Gallego-Martínez A, Alegre-Ituarte V, García-Medina JJ, Pastor-Idoate S, Sellés-Navarro I, Vila-Arteaga J, et al. Oxidative Stress Mediates Epigenetic Modifications and the Expression of miRNAs and Genes Related to Apoptosis in Diabetic Retinopathy Patients. Journal of Clinical Medicine. 2024; 13(1):74. https://doi.org/10.3390/jcm13010074
Chicago/Turabian StyleKaram-Palos, Sarah, Irene Andrés-Blasco, Cristina Campos-Borges, Vicente Zanón-Moreno, Alex Gallego-Martínez, Victor Alegre-Ituarte, Jose J. García-Medina, Salvador Pastor-Idoate, Inmaculada Sellés-Navarro, Jorge Vila-Arteaga, and et al. 2024. "Oxidative Stress Mediates Epigenetic Modifications and the Expression of miRNAs and Genes Related to Apoptosis in Diabetic Retinopathy Patients" Journal of Clinical Medicine 13, no. 1: 74. https://doi.org/10.3390/jcm13010074
APA StyleKaram-Palos, S., Andrés-Blasco, I., Campos-Borges, C., Zanón-Moreno, V., Gallego-Martínez, A., Alegre-Ituarte, V., García-Medina, J. J., Pastor-Idoate, S., Sellés-Navarro, I., Vila-Arteaga, J., Lleó-Perez, A. V., & Pinazo-Durán, M. D. (2024). Oxidative Stress Mediates Epigenetic Modifications and the Expression of miRNAs and Genes Related to Apoptosis in Diabetic Retinopathy Patients. Journal of Clinical Medicine, 13(1), 74. https://doi.org/10.3390/jcm13010074