The Efficacy and Safety of Nivolumab Plus mFOLFOX6 in Gastric Cancer with Severe Peritoneal Metastasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Treatments
2.3. Assessments and Statistical Analysis
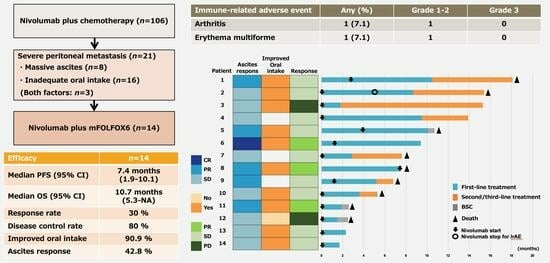
3. Results
3.1. Patient Characteristics and Treatment Exposure
3.2. Efficacy
3.3. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer. Globocan 2020. Cancer Today. 2021. Available online: http://gco.iarc.fr/today (accessed on 19 June 2023).
- Research FfPoC. Cancer Statistics in Japan. 2022. Available online: https://ganjoho.jp/public/qa_links/report/statistics/2022_en.html (accessed on 19 June 2023).
- International Agency for Research on Cancer. Helicobacter pylori. IARC Monographs. 2021. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100B-15.pdf (accessed on 19 June 2023).
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef]
- GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Abbasi, S.Y.; El Taani, H.E.; Saad, A.; Badheeb, A.; Addasi, A. Advanced gastric cancer in Jordan from 2004 to 2008: A study of epidemiology and outcomes. Gastrointest. Cancer Res. 2011, 4, 122–127. [Google Scholar] [PubMed]
- Thomassen, I.; van Gestel, Y.R.; van Ramshorst, B.; Luyer, M.D.; Bosscha, K.; Nienhuijs, S.W.; Lemmens, V.E.; de Hingh, I.H. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int. J. Cancer. 2014, 134, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Boku, N.; Yamamoto, S.; Fukuda, H.; Shirao, K.; Doi, T.; Sawaki, A.; Koizumi, W.; Saito, H.; Yamaguchi, K.; Takiuchi, H.; et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: A randomized phase 3 study. Lancet Oncol. 2009, 10, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, W.; Narahara, H.; Hara, T.; Takagane, A.; Akiya, T.; Takagi, M.; Miyashita, K.; Nishizaki, T.; Kobayashi, O.; Takiyama, W.; et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008, 9, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Higuchi, K.; Nishikawa, K.; Gotoh, M.; Fuse, N.; Sugimoto, N.; Nishina, T.; Amagai, K.; Chin, K.; Niwa, Y.; et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann. Oncol. 2015, 26, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Shirao, K.; Boku, N.; Yamada, Y.; Yamaguchi, K.; Doi, T.; Goto, M.; Nasu, J.; Denda, T.; Hamamoto, Y.; Takashima, A.; et al. Randomized phase III study of 5-fluorouracil continuous infusion vs. sequential methotrexate and 5-fluorouracil therapy in far advanced gastric cancer with peritoneal metastasis (JCOG0106). Jpn. J. Clin. Oncol. 2013, 43, 972–980. [Google Scholar] [CrossRef]
- Iwasa, S.; Goto, M.; Yasui, H.; Nishina, T.; Takahari, D.; Nakayama, N.; Taira, K.; Kusaba, H.; Fuse, N.; Hironaka, S.; et al. Multicenter feasibility study of combination therapy with fluorouracil, leucovorin and paclitaxel (FLTAX) for peritoneal disseminated gastric cancer with massive ascites or inadequate oral intake. Jpn. J. Clin. Oncol. 2012, 42, 787–793. [Google Scholar] [CrossRef]
- Nakajima, T.E.; Yamaguchi, K.; Boku, N.; Hyodo, I.; Mizusawa, J.; Hara, H.; Nishina, T.; Sakamoto, T.; Shitara, K.; Shinozaki, K.; et al. Randomized phase II/III study of 5-fluorouracil/l-leucovorin versus 5-fluorouracil/l-leucovorin plus paclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric. Cancer 2020, 23, 677–688. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.H.; Kim, H.J.; Jang, H.J.; Kim, J.B.; Kim, J.W.; Jung, S.Y.; Kim, B.C.; Yang, D.H.; Park, S.; et al. Oxaliplatin, 5-fluorouracil and leucovorin (modified FOLFOX-6) as first-line chemotherapy for advanced gastric cancer patients with poor performance status. Oncol. Lett. 2012, 3, 425–428. [Google Scholar] [CrossRef]
- Cohen, D.J.; Christos, P.J.; Kindler, H.L.; Catenacci, D.V.T.; Bekaii-Saab, T.B.; Tahiri, S. Vismodegib, a hedgehog pathway inhibitor, combined with FOLFOX for first-line therapy of patients with advanced gastric and gastroesophageal junction carcinoma: A New York cancer Consoritum led phase II randomized study. J. Clin. Oncol. 2013, 31, 4011. [Google Scholar] [CrossRef]
- Shah, M.A.; Cho, J.-Y.; Tan, I.B.; Tebbutt, N.C.; Yen, C.-J.; Kang, A.; Shames, D.S.; Bu, L.; Kang, Y.-K. A randomized phase II study of FOLFOX with or without the MET inhibitor Onartuzumab in advanced adenocarcinoma of the stomach and gastroesophageal junction. Oncologist 2016, 21, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.H.; Bendell, J.C.; Braiteh, F.S.; Firdaus, I.; Philip, P.A.; Cohn, A.L.; Lewis, N.; Anderson, D.M.; Arrowsmith, E.; Schwartz, J.D.; et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: A retrospective study. Int. J. Clin. Oncol. 2011, 16, 57–62. [Google Scholar]
- Oh, S.Y.; Kwon, H.-C.; Lee, S.; Lee, D.M.; Yoo, H.S.; Kim, S.-H.; Jang, J.S.; Kim, M.C.; Jeong, J.-S.; Kim, H.-J. A phase II study of oxaliplatin with low-dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFOX-4) for gastric cancer patients with malignant ascites. Jpn. J. Clin. Oncol. 2007, 37, 930–935. [Google Scholar] [CrossRef]
- Masuishi, T.; Kadowaki, S.; Kondo, M.; Komori, A.; Sugiyama, K.; Mitani, S.; Honda, K.; Narita, Y.; Taniguchi, H.; Ura, T.; et al. FOLFOX as first-line therapy for gastric cancer with severe peritoneal metastasis. Anticancer Res. 2017, 37, 7037–7042. [Google Scholar] [PubMed]
- Osumi, H.; Takahari, D.; Chin, K.; Ogura, M.; Ichimura, T.; Wakatsuki, T.; Suzuki, T.; Ota, Y.; Nakayama, I.; Ooki, A.; et al. Modified FOLFOX6 as a first-line treatment for patients with advanced gastric cancer with massive ascites or inadequate oral intake. Onco. Targets Ther. 2018, 11, 8301–8307. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Chen, L.-T.; Ryu, M.-H.; Oh, D.-Y.; Oh, S.C.; Chung, H.C.; Lee, K.-W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Tesniere, A.; Schlemmer, F.; Boige, V.; Kepp, O.; Martins, I.; Ghiringhelli, F.; Aymeric, L.; Michaud, M.; Apetoh, L.; Barault, L.; et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010, 29, 482–491. [Google Scholar] [CrossRef]
- Vanmeerbeek, I.; Sprooten, J.; De Ruysscher, D.; Tejpar, S.; Vandenberghe, P.; Fucikova, J.; Spisek, R.; Zitvogel, L. Trial watch: Chemotherapy-induced immunogenic cell death in immune-oncology. Oncoimmunology 2020, 9, 1703449. [Google Scholar] [CrossRef]
- Vacchelli, E.; Galluzzi, L.; Fridman, W.H.; Galon, J.; Sautès-Fridman, C.; Tartour, E.; Kroemer, G. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology 2012, 1, 179–188. [Google Scholar] [CrossRef]
- Song, W.; Shen, L.; Wang, Y.; Liu, Q.; Goodwin, T.J.; Li, J.; Dorosheva, O.; Liu, T.; Liu, R.; Huang, L. Synergistic and how adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nat. Commun. 2018, 9, 2237. [Google Scholar] [CrossRef]
- Kim, W.; Chu, T.H.; Nienhüser, H.; Jiang, Z.; Del Portillo, A.; Remotti, H.E.; White, R.A.; Hayakawa, Y.; Tomita, H.; Fox, J.G.; et al. PD-1 signaling promotes tumor-infiltrating myeloid-derived suppressor cells and gastric tumorigenesis in mice. Gastroenterology 2021, 160, 781–796. [Google Scholar] [CrossRef]
- Zhu, H.; Shan, Y.; Ge, K.; Lu, J.; Kong, W.; Jia, C. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell Oncol. 2020, 43, 1203–1214. [Google Scholar] [CrossRef]
- Clinical Practice Guidelines for Peritoneal Malignancy 2021. Japanese Society of Peritoneal Malignancy. Available online: https://www.fukumakuhashu.jp/studygroup.html (accessed on 19 June 2023). (In Japanese).
- Zhao, J.J.; Yap, D.W.T.; Chan, Y.H.; Tan, B.K.J.; Teo, C.B.; Syn, N.L.; Smyth, E.C.; Soon, Y.Y.; Sundar, R. Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J. Clin. Oncol. 2022, 40, 392–402. [Google Scholar] [CrossRef]
- Yoon, H.H.; Jin, Z.; Kour, O.; Fonkoua, L.A.K.; Shitara, K.; Gibson, M.K.; Prokop, L.J.; Moehler, M.; Kang, Y.K.; Shi, Q.; et al. Association of PD-L1 expression and other variables with benefit from immune checkpoint inhibition in advanced gastroesophageal cancer: Systematic review and meta-analysis of 17 phase 3 randomized clinical trials. JAMA Oncol. 2022, 8, 1456–1465. [Google Scholar] [CrossRef]
- Kaneko, T.; Doki, K.; Yamada, T.; Yamamoto, Y.; Moriwaki, T.; Suzuki, Y.; Homma, M. Distribution of therapeutic monoclonal antibodies into ascites in advanced gastric cancer patients with peritoneal metastasis: Case reports and literature review. Cancer Chemother. Pharmacol. 2022, 90, 421–426. [Google Scholar] [CrossRef]
- Fucà, G.; Cohen, R.; Lonardi, S.; Shitara, K.; Elez, M.E.; Fakih, M.; Chao, J.; Klempner, S.J.; Emmett, M.; Jayachandran, P.; et al. Ascites and resistance to immune checkpoint inhibition in dMMR/MSI-H metastatic colorectal and gastric cancer. J. Immunother. Cancer 2022, 10, e004001. [Google Scholar] [CrossRef]
- de Bree, E.; Michelakis, D.; Stamatiou, D.; Romanos, J.; Zoras, O. Pharmacological principles of intraperitoneal and bidirectional chemotherapy. Pleura Peritoneum. 2017, 2, 47–62. [Google Scholar] [CrossRef]
- Chia, D.K.A.; Gwee, Y.X.; Sundar, R. Resistance to systemic immune checkpoint inhibition in the peritoneal niche. J. Immunother. Cancer 2022, 10, e004749. [Google Scholar] [CrossRef]
- Donnenberg, A.D.; Luketich, J.D.; Dhupar, R. Treatment of malignant pleural effusions: The case for localized immunotherapy. J. Immunother. Cancer 2019, 7, 110. [Google Scholar] [CrossRef]
- Buechler, M.B.; Kim, K.-W.; Onufer, E.J.; Williams, J.W.; Little, C.C.; Dominguez, C.X.; Li, Q.; Sandoval, W.; Cooper, J.E.; Harris, C.A.; et al. A stromal niche defined by expression of the transcription factor WT1 mediates programming and homeostasis of cavity-resident macrophages. Immunity 2019, 51, 119–130.e5. [Google Scholar] [CrossRef]
- Park, H.S.; Kwon, W.S.; Park, S.; Jo, E.; Lim, S.J.; Lee, C.-K.; Lee, J.B.; Jung, M.; Kim, H.S.; Beom, S.-H.; et al. Comprehensive immune profiling and immune-monitoring using body fluid of patients with metastatic gastric cancer. J. Immunother. Cancer 2019, 7, 268. [Google Scholar] [CrossRef]
- Küçükköse, E.; Heesters, B.A.; Villaudy, J.; Verheem, A.; Cercel, M.; van Hal, S.; Boj, S.F.; Rinkes, I.H.M.B.; Punt, C.J.A.; Roodhart, J.M.L.; et al. Modeling resistance of colorectal peritoneal metastases to immune checkpoint blockade in humanized mice. J. Immuno Ther. Cancer 2022, 10, e005345. [Google Scholar] [CrossRef]
- Chow, A.; Schad, S.; Green, M.D.; Hellmann, M.D.; Allaj, V.; Ceglia, N.; Zago, G.; Shah, N.S.; Sharma, S.K.; Mattar, M.; et al. Tim-4+ cavity-resident macrophage impair anti-tumor CD8+ T cell immunity. Cancer Cell 2021, 39, 973–988. [Google Scholar] [CrossRef]
- Takashima, A.; Iizumi, S.; Boku, N. Survival after failure of first-line chemotherapy in advanced gastric cancer patients: Differences between Japan and the rest of the world. Jpn. J. Clin. Oncol. 2017, 47, 583–589. [Google Scholar] [CrossRef]
- Harada, D.; Takata, K.; Mori, S.; Kozuki, T.; Takechi, Y.; Moriki, S.; Asakura, Y.; Ohno, T.; Nogami, N. Previous immune checkpoint inhibitor treatment to increase the efficacy of docetaxel and ramucirumab combination chemotherapy. Anticancer. Res. 2019, 39, 4987–4993. [Google Scholar] [CrossRef]
- Chen, D.S.; Hurwitz, H. Combinations of bevacizumab with cancer immunotherapy. Cancer J. 2018, 24, 193–204. [Google Scholar] [CrossRef]
- Zhou, X.; Yao, Z.; Yang, H.; Liang, N.; Zhang, X.; Zhang, F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020, 18, 87. [Google Scholar] [CrossRef]
- Maher, V.E.; Fernandes, L.L.; Weinstock, C.; Tang, S.; Agarwal, S.; Brave, M.; Ning, Y.-M.; Singh, H.; Suzman, D.; Xu, J.; et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J. Clin. Oncol. 2019, 37, 2730–2737. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Dall’Olio, F.G.; Rizzo, A.; Mollica, V.; Massucci, M.; Maggio, I.; Massari, F. Immortal time bias in the association between toxicity and response for immune checkpoint inhibitors: A meta-analysis. Immunotherapy 2021, 13, 257–270. [Google Scholar] [CrossRef]
- Ando, T.; Ueda, A.; Ogawa, K.; Motoo, I.; Kajiura, S.; Nakajima, T.; Hirano, K.; Okumura, T.; Tsukada, K.; Hara, T.; et al. Prognosis of immune-related adverse events in patients with advanced gastric cancer treated with nivolumab or pembrolizumab: A multicenter retrospective analysis. Vivo 2021, 35, 475–482. [Google Scholar] [CrossRef]



| n = 14 | ||
|---|---|---|
| Sex | Male/Female | 8/6 |
| Age (years) | Median (range) | 72 (56–82) |
| Performance status (ECOG) | 0/1/2 | 2/12/0 |
| Disease status | Unresectable/Recurrence | 12/2 |
| History of gastrectomy | −/+ | 11/3 |
| Histologic type | Intestinal/Diffuse | 6/8 |
| HER2 1 status | −/+ | 14/0 |
| MSI 2 status | MSS/MSI-high/NE | 4/0/10 |
| PD-L1 CPS 3 status | <5/≥5/NE | 9/1/4 |
| Ascites | Mild/Moderate/Massive | 6/2/6 |
| Oral intake | Adequate/Inadequate | 3/11 |
| Metastatic sites | Lymph node/Liver/Peritoneal | 11/3/14 |
| Number of metastatic sites | 1−2/≥3 | 10/4 |
| Case | Age | PS | Ascites | Oral Intake | Initial Dose (mg/m2) | Total Course | Relative Dose Intensity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-FU (b) | 5-FU (ci) | L-OHP | mFOLFOX | Nivo | 5-FU (b) | 5-FU (ci) | L-OHP | Nivo | |||||
| 1 | 56 | 1 | Mild | Inadequate | 400 | 2400 | 85 | 18 | 13 | 76.4 | 84.3 | 31 | 72 |
| 2 | 68 | 1 | Mild | Inadequate | 400 | 2400 | 85 | 15 | 7 | 53.3 | 67.7 | 25.5 | 45 |
| 3 | 67 | 1 | Mild | Inadequate | 400 | 2400 | 85 | 4 | 4 | 81.3 | 87.5 | 82.3 | 100 |
| 4 | 68 | 1 | Massive | Adequate | 200 | 1600 | 85 | 10 | 10 | 45 | 60 | 87.6 | 95 |
| 5 | 68 | 1 | Massive | Inadequate | 400 | 2400 | 85 | 9 | 6 | 90.2 | 90.2 | 71.6 | 57 |
| 6 | 74 | 1 | Moderate | Inadequate | 400 | 2400 | 65 | 19 | 18 | 88.2 | 91.2 | 30.9 | 94.7 |
| 7 | 48 | 1 | Massive | Adequate | 400 | 2400 | 85 | 4 | 4 | 87.5 | 88.9 | 87.7 | 91.7 |
| 8 | 72 | 1 | Massive | Inadequate | 200 | 1600 | 50 | 14 | 1 | 47.6 | 63.5 | 56 | 7.1 |
| 9 | 65 | 1 | Massive | Adequate | 400 | 2400 | 85 | 11 | 10 | 100 | 100 | 75.9 | 91 |
| 10 | 77 | 1 | Massive | Inadequate | 400 | 2400 | 85 | 7 | 7 | 75 | 81.7 | 76.2 | 95.2 |
| 11 | 72 | 1 | Moderate | Inadequate | 300 | 2000 | 85 | 5 | 5 | 75 | 83.3 | 100 | 100 |
| 12 | 74 | 1 | Mild | Inadequate | 300 | 2000 | 65 | 2 | 2 | 75 | 83.3 | 76.5 | 100 |
| 13 | 77 | 1 | Mild | Inadequate | 200 | 1600 | 50 | 6 | 6 | 50 | 66.6 | 58.8 | 100 |
| 14 | 82 | 1 | Mild | Inadequate | 300 | 2000 | 65 | 2 | 2 | 75 | 83.3 | 76.5 | 100 |
| CR | PR | SD | PD | Response Rate (%) | Disease Control Rate (%) | |
|---|---|---|---|---|---|---|
| Response for the target lesion (n = 10) | 0 | 3 | 5 | 2 | 30% (3/10) | 80% (8/10) |
| Response for ascites (n = 14) | 1 | 5 | 8 | 0 | 42.8% (6/14) |
| Case | Ascites | Ascites Response | Oral Intake | Improved Oral Intake | Tumor Response | PFS (Months) | 2nd Line | 2nd Line PFS (Months) | OS (Months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Mild | PR | Inadequate | Yes | SD | 10.5 | PTX | 7.6 | 18.1 |
| 2 | Mild | SD | Inadequate | Yes | SD | 8.7 | PTX+Ram | 6.7 | 15.4 |
| 3 | Mild | SD | Inadequate | Yes | PD | 1.8 | PTX+Ram | 13.5 * | 15.3 * |
| 4 | Massive | SD | Adequate | - | nonCR/nonPD | 9.5 | nabPTX+Ram | 4.4 * | 13.9 * |
| 5 | Massive | PR | Inadequate | Yes | SD | 10.1 | - | - | 10.7 |
| 6 | Moderate | CR | Inadequate | Yes | PR | 9.4 * | - | - | 9.4 * |
| 7 | Massive | SD | Adequate | - | nonCR/nonPD | 2.9 | nabPTX+Ram | 4.7 | 7.6 |
| 8 | Massive | PR | Inadequate | Yes | PR | 7.4 | - | - | 7.5 |
| 9 | Massive | PR | Adequate | - | SD | 5.2 | nabPTX | 1.6 | 6.8 |
| 10 | Massive | SD | Inadequate | Yes | SD | 3.6 | PTX+Ram | 1.6 | 5.3 |
| 11 | Moderate | PR | Inadequate | Yes | PR | 1.9 | - | - | 2.6 |
| 12 | Mild | SD | Inadequate | No | PD | 1.5 | - | - | 2.5 |
| 13 | Mild | SD | Inadequate | Yes | SD | 2.3 * | - | - | 2.3 * |
| 14 | Mild | SD | Inadequate | Yes | nonCR/nonPD | 1.7 * | - | - | 1.7 * |
| Grade | Any (%) | 1–2 | 3–4 |
|---|---|---|---|
| Leukopenia | 8 (57.1) | 4 | 4 |
| Neutropenia | 8 (57.1) | 5 | 3 |
| Fever neutropenia | 0 (0) | 0 | 0 |
| Anemia | 9 (64.3) | 8 | 1 |
| Thrombocytopenia | 5 (35.7) | 5 | 0 |
| Nausea | 7 (50.0) | 7 | 0 |
| Vomiting | 4 (28.6) | 4 | 0 |
| Decreased appetite | 9 (64.3) | 8 | 1 |
| Fatigue | 8 (57.1) | 8 | 0 |
| Diarrhea | 2 (14.3) | 1 | 1 |
| Constipation | 4 (28.6) | 4 | 0 |
| Peripheral neuropathy | 9 (64.3) | 9 | 0 |
| Grade | Any (%) | 1–2 | 3 |
|---|---|---|---|
| All | 2 (14.3) | 2 | 0 |
| Arthritis | 1 (7.1) | 1 | 0 |
| Erythema multiforme | 1 (7.1) | 1 | 0 |
| Masuishi et al. [21] | Osumi et al. [22] | This Study | |
|---|---|---|---|
| Regimen | mFOLFOX6 | mFOLFOX6 | Nivolumab+mFOLFOX6 |
| Total number of patients | 10 | 17 | 14 |
| Median age (range) | 64.5 (40–94) | 67 (29–74) | 72 (56–82) |
| PFS (median, months) | 7.5 | 4.2 | 7.4 |
| PFS rate at 6 months (%) | 60.0 | ND | 42.8 |
| OS (median, months) | 13.2 | 8.8 | 10.7 |
| OS rate at 12 months (%) | 50.0 | ND | 28.5 |
| Improved oral intake (%) | 57.0 | 83.0 | 90.9 |
| Ascites response (%) | 78.0 | 50.0 | 42.8 |
| Adverse event (≥Grade3) (%) | |||
| Leukopenia | 0 | 0 | 28.6 |
| Neutropenia | 35.3 | 30 | 21.4 |
| Febrile neutropenia | 5.9 | 0 | 0 |
| Anemia | 0 | 30 | 7.1 |
| Increased AST 1 | 0 | 20 | 0 |
| Increased ALT 2 | 0 | 20 | 0 |
| Vomiting | 5.9 | 0 | 0 |
| Decreased appetite | 5.9 | 0 | 7.1 |
| Diarrhea | 0 | 0 | 7.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, Y.; Ando, T.; Takahashi, N.; Tsukada, K.; Takagi, H.; Goto, Y.; Nakaya, A.; Nakada, N.; Yoshita, H.; Motoo, I.; et al. The Efficacy and Safety of Nivolumab Plus mFOLFOX6 in Gastric Cancer with Severe Peritoneal Metastasis. J. Clin. Med. 2024, 13, 834. https://doi.org/10.3390/jcm13030834
Nakayama Y, Ando T, Takahashi N, Tsukada K, Takagi H, Goto Y, Nakaya A, Nakada N, Yoshita H, Motoo I, et al. The Efficacy and Safety of Nivolumab Plus mFOLFOX6 in Gastric Cancer with Severe Peritoneal Metastasis. Journal of Clinical Medicine. 2024; 13(3):834. https://doi.org/10.3390/jcm13030834
Chicago/Turabian StyleNakayama, Yurika, Takayuki Ando, Naoki Takahashi, Kenichiro Tsukada, Hiroaki Takagi, Yuno Goto, Atsuko Nakaya, Naokatsu Nakada, Hiroki Yoshita, Iori Motoo, and et al. 2024. "The Efficacy and Safety of Nivolumab Plus mFOLFOX6 in Gastric Cancer with Severe Peritoneal Metastasis" Journal of Clinical Medicine 13, no. 3: 834. https://doi.org/10.3390/jcm13030834
APA StyleNakayama, Y., Ando, T., Takahashi, N., Tsukada, K., Takagi, H., Goto, Y., Nakaya, A., Nakada, N., Yoshita, H., Motoo, I., Ueda, A., Ueda, Y., Sakumura, M., Kajiura, S., Ogawa, K., Hosokawa, A., & Yasuda, I. (2024). The Efficacy and Safety of Nivolumab Plus mFOLFOX6 in Gastric Cancer with Severe Peritoneal Metastasis. Journal of Clinical Medicine, 13(3), 834. https://doi.org/10.3390/jcm13030834