Acute Myeloid Leukemia Post Cytotoxic Therapy in Breast Cancer Survivors—Over 23 Years of Single Center Analysis
Abstract
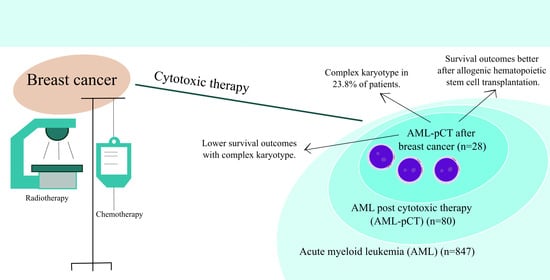
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. AML-pCT after Breast Cancer, Clinical Characteristics
3.2. Molecular and Cytogenetic Characteristics of AML-pCT after Breast Cancer
3.3. Treatment of AML-pCT after Breast Cancer Cytotoxic Therapy
3.4. AML-pCT after Breast Cancer Survival
3.5. Complications of AML-pCT after Breast Cancer Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, A.S.; Niu, J.; Giordano, S.H.; Zhao, H.; Wolff, A.C.; Chavez-MacGregor, M. Acute myeloid leukemia and myelodysplastic syndrome after adjuvant chemotherapy: A population-based study among older breast cancer patients. Cancer 2018, 124, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, A.; Hahn, C.N.; Shah, M.V.; Hiwase, D.K. Role of Germline Predisposition to Therapy-Related Myeloid Neoplasms. Curr. Hematol. Malig. Rep. 2022, 17, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Churpek, J.E.; Marquez, R.; Neistadt, B.; Claussen, K.; Lee, M.K.; Churpek, M.M.; Huo, D.; Weiner, H.; Bannerjee, M.; Godley, L.A.; et al. Inherited mutations in cancer susceptibility genes are common among breast cancer survivors who develop therapy-related leukemia. Cancer 2016, 122, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Font, P. Myelodysplastic syndrome after breast cancer. The challenge of late complications in long-term survivors. Leuk Res. 2016, 49, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Blackford, A.L.; Visvanathan, K.; Rugo, H.S.; Moy, B.; Goldstein, L.J.; Stockerl-Goldstein, K.; Neumayer, L.; Langbaum, T.S.; Theriault, R.L.; et al. Risk of Marrow Neoplasms After Adjuvant Breast Cancer Therapy: The National Comprehensive Cancer Network Experience. J. Clin. Oncol. 2015, 33, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Ok, C.Y.; Patel, K.P.; Garcia-Manero, G.; Routbort, M.J.; Peng, J.; Tang, G.; Goswami, M.; Young, K.H.; Singh, R.; Medeiros, L.J.; et al. TP53 mutation characteristics in therapy-related myelodysplastic syndromes and acute myeloid leukemia is similar to de novo diseases. Hematol. Oncol. 2015, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt Østgård, L.S.; Medeiros, B.C.; Sengeløv, H.; Nørgaard, M.; Andersen, M.K.; Dufva, I.H.; Friis, L.S.; Kjeldsen, E.; Marcher, C.W.; Preiss, B.; et al. Epidemiology and Clinical Significance of Secondary and Therapy-Related Acute Myeloid Leukemia: A National Population-Based Cohort Study. J. Clin. Oncol. 2015, 33, 3641–3649. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef]
- Morsia, E.; McCullough, K.; Joshi, M.; Cook, J.; Alkhateeb, H.B.; Al-Kali, A.; Begna, K.; Elliott, M.; Hogan, W.; Litzow, M.; et al. Venetoclax and hypomethylating agents in acute myeloid leukemia: Mayo Clinic series on 86 patients. Am. J. Hematol. 2020, 95, 1511–1521. [Google Scholar] [CrossRef]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef]
- Adamska, M.; Kowal-Wiśniewska, E.; Przybyłowicz-Chalecka, A.; Barańska, M.; Łojko-Dankowska, A.; Joks, M.; Kanduła, Z.; Jarmuż-Szymczak, M.; Gil, L. Clinical outcomes of therapy-related acute myeloid leukemia: An over 20-year single-center retrospective analysis. Pol. Arch. Intern. Med. 2023, 133, 16344. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Kaplan, H.G.; Malmgren, J.A.; Li, C.I.; Calip, G.S. Age related risk of myelodysplastic syndrome and acute myeloid leukemia among breast cancer survivors. Breast Cancer Res. Treat. 2013, 142, 629–636. [Google Scholar] [CrossRef]
- Guru Murthy, G.S.; Hamadani, M.; Dhakal, B.; Hari, P.; Atallah, E. Incidence and survival of therapy related myeloid neoplasm in United States. Leuk Res. 2018, 71, 95–99. [Google Scholar] [CrossRef]
- Singhal, D.; Wee, L.Y.A.; Kutyna, M.M.; Chhetri, R.; Geoghegan, J.; Schreiber, A.W.; Feng, J.; Wang, P.P.; Babic, M.; Parker, W.T.; et al. The mutational burden of therapy-related myeloid neoplasms is similar to primary myelodysplastic syndrome but has a distinctive distribution. Leukemia 2019, 33, 2842–2853. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Song, H.; Kim, S.M.; Kim, S.; Kwon, S.R.; Lee, Y.E.; Jeong, D.; Park, J.H.; Kwon, S.; Yun, H.; et al. Analysis of clinical and genomic profiles of therapy-related myeloid neoplasm in Korea. Hum. Genom. 2023, 17, 13. [Google Scholar] [CrossRef]
- Hulegårdh, E.; Nilsson, C.; Lazarevic, V.; Garelius, H.; Antunovic, P.; Rangert Derolf, Å.; Möllgård, L.; Uggla, B.; Wennström, L.; Wahlin, A.; et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: A report from the Swedish Acute Leukemia Registry. Am. J. Hematol. 2015, 90, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Fianchi, L.; Pagano, L.; Piciocchi, A.; Candoni, A.; Gaidano, G.; Breccia, M.; Criscuolo, M.; Specchia, G.; Maria Pogliani, E.; Maurillo, L.; et al. Characteristics and outcome of therapy-related myeloid neoplasms: Report from the Italian network on secondary leukemias. Am. J. Hematol. 2015, 90, E80–E85. [Google Scholar] [CrossRef] [PubMed]
- Craig, B.M.; Rollison, D.E.; List, A.F.; Cogle, C.R. Underreporting of myeloid malignancies by United States cancer registries. Cancer Epidemiol. Biomark. Prev. 2012, 21, 474–481. [Google Scholar] [CrossRef]
- Takahashi, K.; Wang, F.; Kantarjian, H.; Doss, D.; Khanna, K.; Thompson, E.; Zhao, L.; Patel, K.; Neelapu, S.; Gumbs, C.; et al. Preleukaemic clonal haemopoiesis and risk of therapy-related myeloid neoplasms: A case-control study. Lancet Oncol. 2017, 18, 100–111. [Google Scholar] [CrossRef]
- Petrone, G.; Gaulin, C.; Derkach, A.; Kishtagari, A.; Robson, M.E.; Parameswaran, R.; Stein, E.M. Routine clinical parameters and laboratory testing predict therapy-related myeloid neoplasms after treatment for breast cancer. Haematologica 2022, 108, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.L.; Jiang, Y.Z.; Shao, Z.M. Survival and chemotherapy-related risk of second primary malignancy in breast cancer patients: A SEER-based study. Int. J. Clin. Oncol. 2019, 24, 934–940. [Google Scholar] [CrossRef]
- Morton, L.M.; Dores, G.M.; Tucker, M.A.; Kim, C.J.; Onel, K.; Gilbert, E.S.; Fraumeni, J.F., Jr.; Curtis, R.E. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975–2008. Blood 2013, 121, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Curtis, R.E.; Boice, J.D., Jr.; Stovall, M.; Bernstein, L.; Greenberg, R.S.; Flannery, J.T.; Schwartz, A.G.; Weyer, P.; Moloney, W.C.; Hoover, R.N. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N. Engl. J. Med. 1992, 326, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Nardi, V.; Winkfield, K.M.; Ok, C.Y.; Niemierko, A.; Kluk, M.J.; Attar, E.C.; Garcia-Manero, G.; Wang, S.A.; Hasserjian, R.P. Acute myeloid leukemia and myelodysplastic syndromes after radiation therapy are similar to de novo disease and differ from other therapy-related myeloid neoplasms. J. Clin. Oncol. 2012, 30, 2340–2347. [Google Scholar] [CrossRef]
- Khalife-Hachem, S.; Saleh, K.; Pasquier, F.; Willekens, C.; Tarabay, A.; Antoun, L.; Grinda, T.; Castilla-Llorente, C.; Duchmann, M.; Quivoron, C.; et al. Molecular Landscape of Therapy-related Myeloid Neoplasms in Patients Previously Treated for Gynecologic and Breast Cancers. HemaSphere 2021, 5, e632. [Google Scholar] [CrossRef]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef]
- Bănescu, C.; Tripon, F.; Muntean, C. The Genetic Landscape of Myelodysplastic Neoplasm Progression to Acute Myeloid Leukemia. Int. J. Mol. Sci. 2023, 24, 5734. [Google Scholar] [CrossRef]
- Nabergoj, M.; Mauff, K.; Beelen, D.; Ganser, A.; Kröger, N.; Stölzel, F.; Finke, J.; Passweg, J.; Cornelissen, J.; Schub, N.; et al. Allogeneic hematopoietic cell transplantation in patients with therapy-related myeloid neoplasm after breast cancer: A study of the Chronic Malignancies Working Party of the EBMT. Bone Marrow Transplant. 2022, 57, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Litzow, M.R.; Tarima, S.; Pérez, W.S.; Bolwell, B.J.; Cairo, M.S.; Camitta, B.M.; Cutler, C.S.; de Lima, M.; Dipersio, J.F.; Gale, R.P. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood 2010, 115, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Metheny, L.; Callander, N.S.; Hall, A.C.; Zhang, M.J.; Bo-Subait, K.; Wang, H.L.; Agrawal, V.; Al-Homsi, A.S.; Assal, A.; Bacher, U.; et al. Allogeneic Transplantation to Treat Therapy-Related Myelodysplastic Syndrome and Acute Myelogenous Leukemia in Adults. Transplant. Cell. Ther. 2021, 27, 923.e1–923.e12. [Google Scholar] [CrossRef] [PubMed]
- Kolonen, A.; Sinisalo, M.; Huttunen, R.; Syrjänen, J.; Aittoniemi, J.; Huhtala, H.; Sankelo, M.; Rintala, H.; Räty, R.; Jantunen, E.; et al. Bloodstream infections in acute myeloid leukemia patients treated according to the Finnish Leukemia Group AML-2003 protocol—A prospective nationwide study. Infect. Dis. 2017, 49, 799–808. [Google Scholar] [CrossRef]
- Czyżewski, K.; Styczyński, J.; Giebel, S.; Frączkiewicz, J.; Salamonowicz, M.; Zając-Spychala, O.; Zaucha-Prażmo, A.; Drozd-Sokołowska, J.; Waszczuk-Gajda, A.; Dybko, J.; et al. Age-dependent determinants of infectious complications profile in children and adults after hematopoietic cell transplantation: Lesson from the nationwide study. Ann. Hematol. 2019, 98, 2197–2211. [Google Scholar] [CrossRef]

| Lp. | Age at BC Diagnosis | BC: Germline Mutational Status | BC Chemotherapy (Details) | BC Radiotherapy (Details) | BC Surgery (Details) | BC Hormone Therapy | Latency Time (years) | AML Post MDS | Other Neoplasm (Details of Treatment) | AML Cytogenetics | AML Molecular Biology Results | AML Treatment | Months of OS (Death/ Alive) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | not tested | yes (A + C, TAX) | yes | yes (mastectomy) | no | 5 | yes | no | trisomy 8 | FLT3-TKD mutation | CTH, alloHCT | 19 (alive) |
| 2 | 44 | no data | yes (A + C) | yes (52.5 Gy) | yes (BCS) | yes | 8 | no | no | 46XX, del(7) (q22) [15] | not performed | CTH, alloHCT | 133 (alive) |
| 3 | 43 | no data | yes | yes (50–60 Gy with cobalt) | yes (Halsted) | yes | 37 | yes | no | not performed | not performed | AZA | 34 (alive) |
| 4 | 46 | no | yes (4× E + C, 11× TAX) | no | yes (mastectomy) | yes | 2 | no | no | 46, XX, t (?6;11) (?q27;q23) [11]/46,XX [2] | t (9;11); KMT2A::MLLT3 | CTH | 3 (death) |
| 5 | 48 | no | no | yes (52.5 Gy) | yes (BCS) | yes | 7 | no | second breast cancer (BCS and radiotherapy) | metaphases not analyzable | not performed | CTH, alloHCT | 18 (alive) |
| 6 | 38 | yes; positive: PALB2 c.509_510delGA p. (Arg170fs), CHEK2 c.470T > C p. (Ile157Thr), (negative: BRCA1, BRCA2, TP53) | yes (1× A + C, 3× E + C) | no | yes (mastectomy + prophylactic mastectomy and hysterectomy) | yes | 5 | no | no | 45, X, −X, t(8;21) (q22;q22) [9]/46, XX, t(8;21) (q22;q22) [2] | C-KIT mutation, t (8;21); RUNX1::RUNX1T1 | CTH, alloHCT | 24 (alive) |
| 7 | 37 | no | yes (4× A + C) | yes (56 Gy) | yes (BCS) | yes | 2 | no | no | inv(16); CBFB::MYH11 | deletion of chromosome 17p13 | CTH, alloHCT | 171 (alive) |
| 8 | 41 | no data | yes (4× A + C, 4× TAC) | yes | yes (mastectomy) | 5 | no | no | metaphases not analyzable | deletion of chromosome 17p13 | CTH, alloHCT | 9 (death) | |
| 9 | 53 | no | no | yes (52.5 Gy) | yes (BCS) | yes | 5 | no | no | 47, XX, +8 [12]/46,XX [1] | FLT3-ITD mutation | CTH | 5 (alive) |
| 10 | 50 | yes: positive: CHEK2 c.470T > C p. (Ile157Thr), (negative: BRCA1, BRCA2) | yes (5× A + C, 4× TAC) | yes (50 Gy) | yes (mastectomy) | yes | 5 | yes | no | 46, XX, t(15;17) (q24;q21) [16]/46, XX, del (7) (q36), t(15;17) (q24;q21) [3]/46, XX [1] | t (15;17); PML::RARA | All-trans retinoic acid, arsenic trioxide with CTH | 81 (alive) |
| 11 | 55 | not tested | yes (6× A + C) | no | yes (mastectomy) | no | 7 | no | no | 45, X, −X, t (8;21) (q22;q22) [17]/46, XX, t(8;21) (q22;q22) [1] | t (8;21); RUNX1::RUNX1T1 | CTH | 104 (alive) |
| 12 | 60 | no data | yes (4× A + C, 12× TAX) | yes (50 Gy) | yes (BCS, SNB) | no | 3 | yes | no | complex karyotype | t (2;11); KMT2A rearranged | CTH | 20 (death) |
| 13 | 49 | not analyzed | yes | yes | yes (mastectomy) | no | 20 | no | uterine cancer (surgery, radiotherapy-brachytherapy, chemotherapy), malignant melanoma (surgery) | 50~52, XX, +X [3], +1 [3], +6 [3], +9 [3], +10 [3], +11 [3], −13 [3], +21 [2], +mar1 [1], +mar2 [2], +mar3 [1] [cp3]/46, XX [5] | not performed | AZA + VEN | 5 (alive) |
| 14 | 71 | no data | no | yes (40 Gy) | yes (BCS) | yes | 3 | no | no | metaphases not analyzable | not performed | AZA | 11 (death) |
| Patients’ Characteristics | AML-pCT after Breast Cancer (n = 28) |
|---|---|
| Age at AML-pCT diagnosis, years | 57.5 (50.5–64.5) |
| Age at breast cancer diagnosis, years | 49.0 (43.0–55.0) |
| Latency time, years | 5.0 (4.0–7.0) |
| Race: white | 28 |
| Primary cytotoxic therapy: | |
| Chemotherapy | 7 |
| Radiotherapy | 9 |
| Both | 12 |
| Laboratory parameters: | |
| WBC, G/L | 2.3 (1.2–16.4) |
| NEU, G/L | 0.8 (0.2–2.5) |
| HGB, mmol/L, | 5.6 (0.9) |
| PLT, G/L | 86.0 (29.3–146.8) |
| BM blasts, % | 41.0 (26.0–80.0) |
| AML-pCT post BC subtypes | |
| APL-pCT post BC | 1 |
| AML-pCT-MR post BC | 6 |
| Cytogenetic or Molecular Marker | n/N% |
|---|---|
| Cytogenetic assessment | |
| Metaphases not analyzable | 4/25 (16.0) |
| Metaphases analyzable | 21/25 (84.0) |
| Normal karyotype | 2/21 (9.5) |
| Cytogenetic abnormalities | 19/21 (90.5) |
| Deletion of chromosome 17p13 | 5/21 (23.8) |
| Complex karyotype a | 4/21 (19.0) |
| Deletion of chromosome 5 | 3/21 (14.3) |
| Monosomal karyotype ᵇ | 2/21 (9.5) |
| t (8;21); RUNX1::RUNX1T1 | 2/21 (9.5) |
| Deletion of chromosome 7 | 2/21 (9.5) |
| t (15;17); PML::RARA | 1/21 (4.8) |
| t (v;11q23.3); KMT2A rearranged | 1/21 (4.8) |
| inv (16) or t (16;16); CBFB::MYH11 | 1/21 (4.8) |
| DNA sequence variants | |
| FLT3—ITD (cDNA) | 3/17 (17.6) |
| NPM1 | 2/14 (14.3) |
| TP53 | 1/12 (8.3) |
| FLT3—TKD (D835) (cDNA) | 1/17 (5.9) |
| C-KIT | 1/1 |
| ELN 2022 genetic risk stratification [14] | |
| Adverse | 13/24 (54.2) |
| Intermediate | 8/24 (33.3) |
| Favorable | 3/24 (12.5) |
| Treatment Characteristics | AML-pCT after Breast Cancer (n = 28) |
|---|---|
| Type of treatment: | |
| Palliative care | 3 |
| Non-intensive therapy | 5 |
| Azacitidine | 2 |
| Azacitidine with venetoclax | 3 |
| Intensive treatment | 20 |
| Intensive chemotherapy | 19 |
| All-trans retinoic acid, arsenic trioxide with chemotherapy | 1 |
| AlloHCT | 12 |
| Targeted therapy with midostaurin | 2 |
| Treatment with 1st induction: | |
| Cytarabine and daunorubicin | 16 |
| Cytarabine, daunorubicin and cladribine | 3 |
| Complete remission | 13 |
| Without complete remission | 5 |
| Incomplete hematologic recovery | 1 |
| NEU recovery (0.5 G/L), days | 28.1 (19.4) |
| No NEU recovery, n | 1 |
| PLT recovery (>50 G/L), days | 25.9 (17.7) |
| No PLT recovery, n | 4 |
| AlloHCT | |
| Identical sibling | 1 |
| Matched unrelated donor | 11 |
| NEU recovery (0.5 G/L), days - | 16.91 (3.51) |
| PLT recovery (>50 G/L), days - | 18.0 (14.0–24.0) |
| AlloHCT in CR1 | 9 |
| AlloHCT in CR2, CR3 or active disease | 3 |
| Stem cell source: peripheral blood | 12 |
| Time from breast cancer diagnosis to alloHCT, years | 5.8 (5.2–7.4) |
| Time from AML-pCT diagnosis to alloHCT, months | 9.5 (8.0–11.0) |
| Myeloablative conditioning | 3 |
| Reduced-intensity conditioning | 9 |
| Factor | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR [95%, CI] | p Value | AHR [95%, CI] | p Value | |
| Latency time, years | 0.90 [0.74–1.09] | 0.29 | ||
| Age at t-AML diagnosis | ||||
| ≥65 years old | 2.01 [0.58–6.92] | 0.27 | ||
| <65 years old | 1 (ref) | |||
| t-AML treatment | ||||
| Palliative | 1 (ref) | 1 (ref) | ||
| Intensive chemotherapy | 0.35 [0.08–1.53] | 0.16 | 0.37 [0.07–1.74] | 0.21 |
| AlloHCT | 0.12 [0.03–0.56] | 0.007 | 0.07 [0.01–0.80] | 0.033 |
| Non-intensive treatment | 0.15 [0.02–1.49] | 0.11 | 0.0 [0.00–10.14] | 0.98 |
| Type of previous cytotoxic therapy | ||||
| Radiotherapy | 1 (ref) | |||
| Chemotherapy | 1.10 [0.25–4.93] | 0.90 | ||
| Radiotherapy + chemotherapy | 1.57 [0.43–5.76] | 050 | ||
| Laboratory parameters at diagnosis | ||||
| BM blasts, % | 1.00 [0.98–1.02] | 0.82 | ||
| WBC, G/L | 1.00 [0.99–1.01] | 0.82 | ||
| NEU, G/L | 0.98 [0.86–1.11] | 0.73 | ||
| HGB, mmol/L | 0.51 [0.24–1.10] | 0.09 | ||
| PLT, G/L | 1.00 [0.99–1.00] | 0.47 | ||
| 2022 ELN genetic risk category [14] | ||||
| Favorable | 0.00 [9.06–1.72] | 0.96 | 0.0 [1.61–79.90] | 0.96 |
| Intermediate | 1 (ref) | 1 (ref) | ||
| Adverse | 7.19 [0.91–56.48] | 0.06 | 1.01 [0.08–13.59] | 0.99 |
| Cytogenetic abnormalities | ||||
| Complex karyotype | 3.33 [0.93–11.92] | 0.06 | 2.99 [0.67–13.48] | 0.15 |
| Non-complex karyotype | 1 (ref) | 1 (ref) | ||
| Treatment with first induction: | ||||
| Complete remission | 0.64 [0.17–2.39] | 0.51 | ||
| Without complete remission | 1 (ref) | |||
| AML-pCT post BC subtype: | ||||
| AML-pCT | 1 (ref) | |||
| AML-pCT-MR | 1.82 [0.55–6.03] | 0.33 | ||
| AML-pCT post BC intensively treated: | ||||
| AML-pCT | 1 (ref) | |||
| AML-pCT-MR | 5.95 [1.30–27.33] | 0.022 | ||
| AlloHCT (n = 12) | Intensive Chemotherapy (n = 20) | ||
|---|---|---|---|
| Complication | n, % | Complication | n, % |
| Renal toxicity a (total) | 12 (100.0) | Hepatotoxicity a | 10 (71.4) |
| 0–30 days after alloHCT | 7 (58.3) | grade 1/grade 2/grade 3/grade 4 | 5/3/1/1 |
| grade 1/grade 2/grade 3 | 3/3/1 | Renal toxicity a | 5 (35.7) |
| 30–100 days after alloHCT | 10 (90.1) | grade 1/grade 2/grade 3 | 5/0/0 |
| grade 1/grade 2/grade 3 | 5/4/1 | Cardiotoxicity a | 3 (21.4) |
| Hepatotoxicity a (total) | 11 (91.7) | grade 1/grade 2/grade 3 | 3/0/0 |
| 0–30 days after alloHCT | 9 (75.0) | Neurotoxicity | 4 (28.6) |
| grade 1/grade 2/grade 3 | 4/3/2 | Deep vein thrombosis | 2 (14.3) |
| 30–100 days after alloHCT | 9 (81.8) | Psychiatric | 2 (14.3) |
| grade 1/grade 2/grade 3 | 8/0/1 | Iatrogenic adverse events | 1 (7.1) |
| Cardiotoxicity a | 3 (25.0) | ||
| grade 1/grade 2/grade 3 | 1/0/0 | ||
| Hemorrhagic cystitis | 1 (8.3) | ||
| Pulmonary fibrosis | 1 (8.3) | No data | 6 (30.0) |
| Type of Infections | AlloHCT (n = 12) | Intensive Chemotherapy (n = 20) | ||
|---|---|---|---|---|
| ≤30 Days | >30 Days | Total | ||
| Fever of unknown origin | 10 (100.0) | 2 (20.0) | 10 (100.0) | 14 (93.3) |
| Bacterial blood stream infections | 5 (50.0) | 2 (20.0) | 6 (60.0) | 6 (40.0) |
| Gram-negative | 4 (40.0) | 1 (10.0) | 4 (40.0) | 3 (20.0) |
| Escherichia coli | 2 (20.0) | - | 2 (20.0) | 1 (6.7) |
| Klebsiella pneumoniae | 1 (10.0) | 1 (10.0) | 1 (10.0) | 1 (6.7) |
| Stenotrophomonas maltophilia | - | - | - | 1 (6.7) |
| Serratia marcescens | 1 (10.0) | 1 (10.0) | - | |
| Gram-positive | 1 (10.0) | 1 (10.0) | 2 (20.0) | 5 (33.3) |
| Staphylococcus aureus | - | - | - | 2 (13.3) |
| Staphylococcus epidermidis | - | - | - | 1 (6.7) |
| Enterococcus faecium | 1 (10.0) | 1 (10.0) | 2 (20.0) | 1 (6.7) |
| Staphylococcus haemolyticus | - | - | - | 1 (6.7) |
| Viral infections | - | 3 (30.0) | 3 (30.0) | - |
| Cytomegalovirus (CMV) | - | 3 (30.0) | 3 (30.0) | - |
| Fungal infections | - | - | - | - |
| Serum galactomannan | - | 1 (10.0) | 1 (10.0) | - |
| Data unavailable | 2 (20.0) | 2 (20.0) | 2 (20.0) | 5 (26.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamska, M.; Kowal-Wiśniewska, E.; Barańska, M.; Przybyłowicz-Chalecka, A.; Łojko-Dankowska, A.; Joks, M.; Jarmuż-Szymczak, M.; Gil, L. Acute Myeloid Leukemia Post Cytotoxic Therapy in Breast Cancer Survivors—Over 23 Years of Single Center Analysis. J. Clin. Med. 2024, 13, 989. https://doi.org/10.3390/jcm13040989
Adamska M, Kowal-Wiśniewska E, Barańska M, Przybyłowicz-Chalecka A, Łojko-Dankowska A, Joks M, Jarmuż-Szymczak M, Gil L. Acute Myeloid Leukemia Post Cytotoxic Therapy in Breast Cancer Survivors—Over 23 Years of Single Center Analysis. Journal of Clinical Medicine. 2024; 13(4):989. https://doi.org/10.3390/jcm13040989
Chicago/Turabian StyleAdamska, Monika, Ewelina Kowal-Wiśniewska, Marta Barańska, Anna Przybyłowicz-Chalecka, Anna Łojko-Dankowska, Monika Joks, Małgorzata Jarmuż-Szymczak, and Lidia Gil. 2024. "Acute Myeloid Leukemia Post Cytotoxic Therapy in Breast Cancer Survivors—Over 23 Years of Single Center Analysis" Journal of Clinical Medicine 13, no. 4: 989. https://doi.org/10.3390/jcm13040989
APA StyleAdamska, M., Kowal-Wiśniewska, E., Barańska, M., Przybyłowicz-Chalecka, A., Łojko-Dankowska, A., Joks, M., Jarmuż-Szymczak, M., & Gil, L. (2024). Acute Myeloid Leukemia Post Cytotoxic Therapy in Breast Cancer Survivors—Over 23 Years of Single Center Analysis. Journal of Clinical Medicine, 13(4), 989. https://doi.org/10.3390/jcm13040989