Current Status of and Future Prospects for Drug-Eluting Stents and Scaffolds in Infrapopliteal Arteries
Abstract
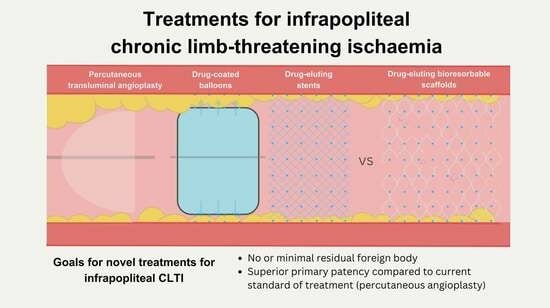
:1. Introduction
2. Treatment of Infrapopliteal Disease in CLTI
2.1. Balloon Angioplasty and Bare Metal Stents
2.2. Drug-Eluting Stents
2.3. Drug-Coated Balloons
2.4. Tack Endovascular System
2.5. Drug-Eluting Bioresorbable Scaffolds
3. Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, Regional, and National Prevalence and Risk Factors for Peripheral Artery Disease in 2015: An Updated Systematic Review and Analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. J. Vasc. Surg. 2019, 69, 3S–125S.e40. [Google Scholar] [CrossRef]
- Mustapha, J.A.; Katzen, B.T.; Neville, R.F.; Lookstein, R.A.; Zeller, T.; Miller, L.E.; Jaff, M.R. Determinants of Long-Term Outcomes and Costs in the Management of Critical Limb Ischemia: A Population-Based Cohort Study. J. Am. Heart Assoc. 2018, 7, e009724. [Google Scholar] [CrossRef]
- Bradbury, A.W.; Moakes, C.A.; Popplewell, M.; Meecham, L.; Bate, G.R.; Kelly, L.; Chetter, I.; Diamantopoulos, A.; Ganeshan, A.; Hall, J.; et al. A Vein Bypass First versus a Best Endovascular Treatment First Revascularisation Strategy for Patients with Chronic Limb Threatening Ischaemia Who Required an Infra-Popliteal, with or without an Additional More Proximal Infra-Inguinal Revascularisation Procedure to Restore Limb Perfusion (BASIL-2): An Open-Label, Randomised, Multicentre, Phase 3 Trial. Lancet 2023, 401, 1798–1809. [Google Scholar] [CrossRef]
- Scheinert, D.; Katsanos, K.; Zeller, T.; Koppensteiner, R.; Commeau, P.; Bosiers, M.; Krankenberg, H.; Baumgartner, I.; Siablis, D.; Lammer, J.; et al. A Prospective Randomized Multicenter Comparison of Balloon Angioplasty and Infrapopliteal Stenting with the Sirolimus-Eluting Stent in Patients with Ischemic Peripheral Arterial Disease: 1-Year Results from the Achilles Trial. J. Am. Coll. Cardiol. 2012, 60, 2290–2295. [Google Scholar] [CrossRef]
- Bosiers, M.; Scheinert, D.; Peeters, P.; Torsello, G.; Zeller, T.; Deloose, K.; Schmidt, A.; Tessarek, J.; Vinck, E.; Schwartz, L.B. Randomized Comparison of Everolimus-Eluting versus Bare-Metal Stents in Patients with Critical Limb Ischemia and Infrapopliteal Arterial Occlusive Disease. J. Vasc. Surg. 2012, 55, 390–398. [Google Scholar] [CrossRef]
- Siablis, D.; Kitrou, P.M.; Spiliopoulos, S.; Katsanos, K.; Karnabatidis, D. Paclitaxel-Coated Balloon Angioplasty versus Drug-Eluting Stenting for the Treatment of Infrapopliteal Long-Segment Arterial Occlusive Disease: The IDEAS Randomized Controlled Trial. JACC Cardiovasc. Interv. 2014, 7, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Spreen, M.I.; Martens, J.M.; Hansen, B.E.; Knippenberg, B.; Verhey, E.; Van Dijk, L.C.; De Vries, J.P.P.M.; Vos, J.A.; De Borst, G.J.; Vonken, E.J.P.A.; et al. Percutaneous Transluminal Angioplasty and Drug-Eluting Stents for Infrapopliteal Lesions in Critical Limb Ischemia (PADI) Trial. Circ. Cardiovasc. Interv. 2016, 9, e002376. [Google Scholar] [CrossRef] [PubMed]
- Rastan, A.; Brechtel, K.; Krankenberg, H.; Zahorsky, R.; Tepe, G.; Noory, E.; Schwarzwälder, U.; MacHarzina, R.; Schwarz, T.; Bürgelin, K.; et al. Sirolimus-Eluting Stents for Treatment of Infrapopliteal Arteries Reduce Clinical Event Rate Compared to Bare-Metal Stents: Long-Term Results from a Randomized Trial. J. Am. Coll. Cardiol. 2012, 60, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, P.J.; Adams, G.L.; Schmidt, A.; Lichtenberg, M.; Wissgott, C.; Armstrong, E.J.; Hertting, K.; Cardenas, J.; Lichtenberg, M.; Wissgott, C.; et al. Twelve-Month Results of Tack-Optimized Balloon Angioplasty Using the Tack Endovascular System in Below-the-Knee Arteries (TOBA II BTK). J. Endovasc. Ther. 2020, 27, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.L.; Lichtenberg, M.; Wissgott, C.; Schmidt, A.; Tarra, T.; Matricardi, S.; Geraghty, P.J. Twenty-Four Month Results of Tack-Optimized Balloon Angioplasty Using the Tack Endovascular System in Below-the-Knee Arteries. J. Endovasc. Ther. 2023, 30, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Varcoe, R.L.; Parikh, S.A.; DeRubertis, B.G.; Jones-McMeans, J.M.; Teraphongphom, N.T.; Wang, J.; Kolluri, R.; Weinberg, I.; Holden, A.H.; Garcia-Garcia, H.M.; et al. Evaluation of an Infrapopliteal Drug-Eluting Resorbable Scaffold: Design Methodology for the LIFE-BTK Randomized Controlled Trial. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100964. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence Peripheral Arterial Disease: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/cg147 (accessed on 24 December 2023).
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e686–e725. [Google Scholar] [CrossRef] [PubMed]
- Nordanstig, J.; Behrendt, C.-A.; Baumgartner, I.; Belch, J.; Bäck, M.; Fitridge, R.; Hinchliffe, R.; Lejay, A.; Mills, J.L.; Rother, U.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Asymptomatic Lower Limb Peripheral Arterial Disease and Intermittent Claudication. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 9–96. [Google Scholar] [CrossRef]
- Chioncel, V.; Brezeanu, R.; Sinescu, C. New Directions in the Management of Peripheral Artery Disease. Am. J. Ther. 2019, 26, e284–e293. [Google Scholar] [CrossRef]
- Giannopoulos, S.; Varcoe, R.L.; Lichtenberg, M.; Rundback, J.; Brodmann, M.; Zeller, T.; Schneider, P.A.; Armstrong, E.J. Balloon Angioplasty of Infrapopliteal Arteries: A Systematic Review and Proposed Algorithm for Optimal Endovascular Therapy. J. Endovasc. Ther. 2020, 27, 547–564. [Google Scholar] [CrossRef]
- Romiti, M.; Albers, M.; Brochado-Neto, F.C.; Durazzo, A.E.S.; Pereira, C.A.B.; De Luccia, N. Meta-Analysis of Infrapopliteal Angioplasty for Chronic Critical Limb Ischemia. J. Vasc. Surg. 2008, 47, 975–981. [Google Scholar] [CrossRef]
- Randon, C.; Jacobs, B.; De Ryck, F.; Vermassen, F. Angioplasty or Primary Stenting for Infrapopliteal Lesions: Results of a Prospective Randomized Trial. Cardiovasc. Interv. Radiol. 2010, 33, 260–269. [Google Scholar] [CrossRef]
- Konijn, L.C.D.; Wakkie, T.; Spreen, M.I.; de Jong, P.A.; van Dijk, L.C.; Wever, J.J.; Veger, H.T.C.; Statius van Eps, R.G.; Mali, W.P.T.M.; van Overhagen, H. 10-Year Paclitaxel Dose-Related Outcomes of Drug-Eluting Stents Treated Below the Knee in Patients with Chronic Limb-Threatening Ischemia (The PADI Trial). Cardiovasc. Interv. Radiol. 2020, 43, 1881–1888. [Google Scholar] [CrossRef]
- Wang, T.-H.; Wang, H.-S.; Soong, Y.-K. Paclitaxel-Induced Cell Death. Cancer 2000, 88, 2619–2628. [Google Scholar] [CrossRef]
- Kaul, U.; Bangalore, S.; Seth, A.; Arambam, P.; Abhaichand, R.K.; Patel, T.M.; Banker, D.; Abhyankar, A.; Mullasari, A.S.; Shah, S.; et al. Paclitaxel-Eluting versus Everolimus-Eluting Coronary Stents in Diabetes. N. Engl. J. Med. 2015, 373, 1709–1719. [Google Scholar] [CrossRef]
- Varcoe, R.L.; Paravastu, S.C.; Thomas, S.D.; Bennett, M.H. The Use of Drug-Eluting Stents in Infrapopliteal Arteries: An Updated Systematic Review and Meta-Analysis of Randomized Trials. Int. Angiol. 2019, 38, 121–135. [Google Scholar] [CrossRef]
- Daemen, J.; Serruys, P.W. Drug-Eluting Stent Update 2007: Part I: A Survey of Current and Future Generation Drug-Eluting Stents: Meaningful Advances or More of the Same? Circulation 2007, 116, 316–328. [Google Scholar] [CrossRef]
- van Overhagen, H.; Nakamura, M.; Geraghty, P.J.; Rao, S.; Arroyo, M.; Soga, Y.; Iida, O.; Armstrong, E.; Nakama, T.; Fujihara, M.; et al. Primary Results of the SAVAL Randomized Trial of a Paclitaxel-Eluting Nitinol Stent versus Percutaneous Transluminal Angioplasty in Infrapopliteal Arteries. Vasc. Med. 2023, 28, 571–580. [Google Scholar] [CrossRef]
- Changal, K.; Patel, M.; Devarasetty, P.P.; Royfman, R.; Veria, S.; Vyas, R.; Mhanna, M.; Patel, N.; Beran, A.; Burket, M.; et al. Drug-Eluting Stents Versus Conventional Endovascular Therapies in Symptomatic Infrapopliteal Peripheral Artery Disease: A Meta-Analysis. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100024. [Google Scholar] [CrossRef]
- Fusaro, M.; Cassese, S.; Ndrepepa, G.; Tepe, G.; King, L.; Ott, I.; Nerad, M.; Schunkert, H.; Kastrati, A. Drug-Eluting Stents for Revascularization of Infrapopliteal Arteries. JACC Cardiovasc. Interv. 2013, 6, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, G.A.; Chalmers, N.; Kanesalingham, K.; Antoniou, S.A.; Schiro, A.; Serracino-Inglott, F.; Smyth, J.V.; Murray, D. Meta-Analysis of Outcomes of Endovascular Treatment of Infrapopliteal Occlusive Disease With Drug-Eluting Stents. J. Endovasc. Ther. 2013, 20, 131–144. [Google Scholar] [CrossRef]
- Katsanos, K.; Spiliopoulos, S.; Diamantopoulos, A.; Karnabatidis, D.; Sabharwal, T.; Siablis, D. Systematic Review of Infrapopliteal Drug-Eluting Stents: A Meta-Analysis of Randomized Controlled Trials. Cardiovasc. Interv. Radiol. 2013, 36, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Ipema, J.; Kum, S.; Huizing, E.; Schreve, M.A.; Varcoe, R.L.; Hazenberg, C.E.; de Vries, J.P.; Ünlü, Ç. A Systematic Review and Meta-Analysis of Bioresorbable Vascular Scaffolds for below-the-Knee Arterial Disease. Int. Angiol. 2021, 40, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Varcoe, R.L.; DeRubertis, B.G.; Kolluri, R.; Krishnan, P.; Metzger, D.C.; Bonaca, M.P.; Shishehbor, M.H.; Holden, A.H.; Bajakian, D.R.; Garcia, L.A.; et al. Drug-Eluting Resorbable Scaffold versus Angioplasty for Infrapopliteal Artery Disease. N. Engl. J. Med. 2024, 390, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Liistro, F.; Angioli, P.; Ventoruzzo, G.; Ducci, K.; Reccia, M.R.; Ricci, L.; Falsini, G.; Scatena, A.; Pieroni, M.; Bolognese, L. Randomized Controlled Trial of Acotec Drug-Eluting Balloon Versus Plain Balloon for Below-the-Knee Angioplasty. JACC Cardiovasc. Interv. 2020, 13, 2277–2286. [Google Scholar] [CrossRef]
- Zeller, T.; Beschorner, U.; Pilger, E.; Bosiers, M.; Deloose, K.; Peeters, P.; Scheinert, D.; Schulte, K.-L.; Rastan, A.; Brodmann, M. Paclitaxel-Coated Balloon in Infrapopliteal Arteries. JACC Cardiovasc. Interv. 2015, 8, 1614–1622. [Google Scholar] [CrossRef]
- Zeller, T.; Baumgartner, I.; Scheinert, D.; Brodmann, M.; Bosiers, M.; Micari, A.; Peeters, P.; Vermassen, F.; Landini, M.; Snead, D.B.; et al. Drug-Eluting Balloon Versus Standard Balloon Angioplasty for Infrapopliteal Arterial Revascularization in Critical Limb Ischemia. J. Am. Coll. Cardiol. 2014, 64, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Al Halabi, S.; Templeton, E.; Heaney, C.; Fadi, S.; Varcoe, R.L.; Van Den Berg, J.C.; Golzar, J.; Al Khaled, N.; Armstrong, E.; Mustapha, J.A. Drug-Coated versus Uncoated Percutaneous Transluminal Angioplasty Balloons for the Treatment of Infrapopliteal Peripheral Artery Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Crit. Limb Ischemia 2022, 2, E11–E16. [Google Scholar]
- Brodmann, M. Results and Interim Safety Analyses at Three Years. In Proceedings of the Amputation Prevention Symposium, Virtual, 12–15 August 2020. [Google Scholar]
- Tang, T.Y.; Yap, C.; Soon, S.X.Y.; Chan, S.L.; Lee, Q.W.S.; Yap, H.Y.; Tay, H.T.L.; Chong, T.T. World’s First Experience Treating TASC II C and D Tibial Occlusive Disease Using the Selution SLR Sirolimus-Eluting Balloon: Six-Month Results from the PRESTIGE Study. J. Endovasc. Ther. 2021, 28, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Lemos, P.A.; Farooq, V.; Takimura, C.K.; Gutierrez, P.S.; Virmani, R.; Kolodgie, F.; Christians, U.; Kharlamov, A.; Doshi, M.; Sojitra, P.; et al. Emerging Technologies: Polymer-Free Phospholipid Encapsulated Sirolimus Nanocarriers for the Controlled Release of Drug from a Stent-plus-Balloon or a Stand-Alone Balloon Catheter. EuroIntervention 2013, 9, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.Y.; Yap, C.J.Q.; Soon, S.X.Y.; Chan, S.L.; Khoo, V.B.X.; Chong, T.T. 12-Months Results from the PRESTIGE Study Using Sirolimus Drug-Eluting Balloons in the Treatment of Complex BTK Tibial Atherosclerotic Lesions in CLTI Patients. Cardiovasc. Revascularization Med. 2022, 43, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Teichgräber, U.; Platzer, S.; Lehmann, T.; Ingwersen, M.; Aschenbach, R.; Beschorner, U.; Scheinert, D.; Zeller, T. Sirolimus-Coated Balloon Angioplasty of Infra-Popliteal Lesions for the Treatment of Chronic Limb-Threatening Ischemia: Study Protocol for the Randomized Controlled LIMES Study. Cardiovasc. Intervent Radiol. 2022, 45, 1716–1724. [Google Scholar] [CrossRef]
- Armstrong, E. CRT-300.3 SELUTION4BTK—A Randomized Clinical Trial Evaluating Selution SLR Sirolimus-Eluting Balloon in the Treatment of Below-the-Knee Lesions in Patients With Chronic Limb-Threatening Ischemia. JACC Cardiovasc. Interv. 2023, 16, S50. [Google Scholar] [CrossRef]
- ClinicalTrials.Gov. SirolimUs CoaTed Balloon for the TrEatment of Below the Knee Arterial Disease (FUTURE-BTK). Available online: https://clinicaltrials.gov/study/NCT04511247 (accessed on 2 March 2024).
- Razavi, M.K.; Mustapha, J.A.; Miller, L.E. Contemporary Systematic Review and Meta-Analysis of Early Outcomes with Percutaneous Treatment for Infrapopliteal Atherosclerotic Disease. J. Vasc. Interv. Radiol. 2014, 25, 1489–1496.e3. [Google Scholar] [CrossRef]
- Phillips. TOBA II BTK Clinical Study 36-Month Summary; Royal Philips (Technology Company): Amsterdam, The Netherlands, 2022. [Google Scholar]
- Varcoe, R.L.; Menting, T.P.; Thomas, S.D.; Lennox, A.F. Long-Term Results of a Prospective, Single-Arm Evaluation of Everolimus-Eluting Bioresorbable Vascular Scaffolds in Infrapopliteal Arteries. Catheter. Cardiovasc. Interv. 2021, 97, 142–149. [Google Scholar] [CrossRef]
- Ellis, S.G.; Kereiakes, D.J.; Metzger, D.C.; Caputo, R.P.; Rizik, D.G.; Teirstein, P.S.; Litt, M.R.; Kini, A.; Kabour, A.; Marx, S.O.; et al. Everolimus-Eluting Bioresorbable Scaffolds for Coronary Artery Disease. N. Engl. J. Med. 2015, 373, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.I.; Schneider, P.A. New Innovations and Devices in the Management of Chronic Limb-Threatening Ischemia. J. Endovasc. Ther. 2020, 27, 524–539. [Google Scholar] [CrossRef] [PubMed]
- Rand, T. MOTIV Bioresorbable BTK Scaffold Pilot Study Preliminary Results through 6 Months. In Proceedings of the Leipzig Interventional Course, Leipzig, Germany, 6–9 June 2022. [Google Scholar]
- ClinicalTrials.gov. MOTIV BTK Randomized Controlled Trial. Available online: https://clinicaltrials.gov/study/NCT05406622 (accessed on 23 December 2023).
- Secemski, E. RESOLV FIH 6-Month Results by Rutherford Classification. In Proceedings of the Vascular InterVentional Advances, Las Vegas, NV, USA, 31 October–2 November 2023. [Google Scholar]
- Banerjee, S.; Jeon-Slaughter, H.; Armstrong, E.J.; Bajzer, C.; Abu-Fadel, M.; Khalili, H.; Prasad, A.; Bou Dargham, B.; Kamath, P.; Addo, T.; et al. Clinical Outcomes and Cost Comparisons of Stent and Non-Stent Interventions in Infrainguinal Peripheral Artery Disease: Insights from the Excellence in Peripheral Artery Disease (XLPAD) Registry. J. Invasive Cardiol. 2019, 31, 1–9. [Google Scholar]
- Jackson-Smith, E.; Zioupos, S.; Banerjee, P. Bioresorbable Vascular Scaffolds versus Conventional Drug-Eluting Stents across Time: A Meta-Analysis of Randomised Controlled Trials. Open Heart 2022, 9, e002107. [Google Scholar] [CrossRef] [PubMed]
- Haude, M.; Wlodarczak, A.; van der Schaaf, R.J.; Torzewski, J.; Ferdinande, B.; Escaned, J.; Iglesias, J.F.; Bennett, J.; Toth, G.G.; Joner, M.; et al. A New Resorbable Magnesium Scaffold for de Novo Coronary Lesions (DREAMS 3): One-Year Results of the BIOMAG-I First-in-Human Study. EuroIntervention 2023, 19, e414–e422. [Google Scholar] [CrossRef] [PubMed]
- Haude, M.; Wlodarczak, A.; van der Schaaf, R.J.; Torzewski, J.; Ferdinande, B.; Escaned, J.; Iglesias, J.F.; Bennett, J.; Toth, G.; Joner, M.; et al. Safety and Performance of the Third-Generation Drug-Eluting Resorbable Coronary Magnesium Scaffold System in the Treatment of Subjects with de Novo Coronary Artery Lesions: 6-Month Results of the Prospective, Multicenter BIOMAG-I First-in-Human Study. EClinicalMedicine 2023, 59, 101940. [Google Scholar] [CrossRef] [PubMed]
- Tepe, G.; Brodmann, M.; Bachinsky, W.; Holden, A.; Zeller, T.; Mangalmurti, S.; Nolte-Ernsting, C.; Virmani, R.; Parikh, S.A.; Gray, W.A. Intravascular Lithotripsy for Peripheral Artery Calcification: Mid-Term Outcomes from the Randomized Disrupt PAD III Trial. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100341. [Google Scholar] [CrossRef]
- Brodmann, M.; Holden, A.; Zeller, T. Safety and Feasibility of Intravascular Lithotripsy for Treatment of Below-the-Knee Arterial Stenoses. J. Endovasc. Ther. 2018, 25, 499–503. [Google Scholar] [CrossRef]
- Losurdo, F.; Ferraresi, R.; Ucci, A.; Zanetti, A.; Clerici, G.; Zambon, A. Association of Infrapopliteal Medial Arterial Calcification with Lower-Limb Amputations in High-Risk Patients: A Systematic Review and Meta-Analysis. Vasc. Med. 2021, 26, 164–173. [Google Scholar] [CrossRef]
| Trial | Drug-Eluting Stent or Scaffold | Control | Participants in Drug-Eluting Device Group n (%) | Mean Lesion Length (mm) | Follow-Up Interval | Primary Endpoint(s) |
|---|---|---|---|---|---|---|
| YUKON BTK [9] | Sirolimus DES | PTA + BMS | 82/161 (51) | 31 ± 9 | DES: 1005 ± 139 days BMS: 1027 ± 123 days | Event-free survival * DES: 65.8% vs. PTA + BMS: 44.6%; p = 0.02 |
| DESTINY [6] | Everolimus DES | PTA + BMS | 74/140 (53) | DES: 15.9 ± 10.2 PTA + BMS: 18.9 ± 10.0 | 12 months | 12-month primary patency (absence of ≥50% restenosis) DES: 85% vs. PTA + BMS 54%; p = 0.0001 |
| ACHILLES [5] | Sirolimus DES | PTA | 99/200 (50) | 26.9 | 12 months | 12-month in-segment binary restenosis by quantitative angiography DES: 22.4 vs. PTA: 41.9%; p = 0.02 |
| PADI [8] | Paclitaxel DES | PTA ± BMS | DES: 74/140 limbs in 137 patients | DES: 21.1 ± 19.3 PTA ± BMS: 23.1 ± 21.8 | 6 months, 12 months, and 24 months | 6-month primary patency (≤50% stenosis on CT angiography) DES: 48.0 vs. PTA ± BMS: 31.5%; p = 0.10 |
| IDEAS [7] | Paclitaxel DES | Paclitaxel DCB | DES: 30 arteries in 27 limbs PCB: 25 arteries in 25 limbs | DES: 127 ± 46.5 PCB: 148 ± 56 | 6 months | 6-month binary restenosis (>50%) DES: 28% vs. PCB: 57.9% p = 0.05 |
| SAVAL [25] | Nitinol (self-expanding) paclitaxel DES | PTA | 130/201 (65) | DES: 68.1 ± 35.2 PTA: 68.7 ± 49.2 | 1, 3, 6, and 12 months | Efficacy: 12-month primary vessel patency DES: 68.0% vs. PTA: 76.0; p = 0.86 Safety: 12-month MAE-free rate DES: 91.6% vs. PTA: 95.3%; p = 0.43 |
| LIFE-BTK [31] | Everolimus-eluting DRS | PTA | 173/261 (66) | DRS: 43 ± 31.8 PTA: 44.8 ± 29.1 | 12 months | Efficacy: 12-month freedom from: above-ankle amputation of target limb, CD-TLR, and binary restenosis BRS: 74% vs. PTA: 44%; p < 0.001 Safety: freedom from MALEs 6 months and POD BRS: 165/170 and PTA: 90/90; p < 0.001 noninferiority |
| Device | Benefits | Limitations |
|---|---|---|
| Percutaneous angioplasty | Cost-effective Repeatable No permanent implant | High rates of elastic recoil and dissection High rates of restenosis |
| Bare metal stent | Rescue following failed angioplasty | Permanent metallic implant Risk of stent fracture |
| Drug-coated balloon | No permanent implant | Mixed evidence for efficacy |
| Drug-eluting stent | Superior primary patency compared with angioplasty and bare metal stenting | Permanent metallic implant Risk of stent fracture Limited to application in short lesions |
| Tack endovascular system | Only device available for use as rescue post-dissection | Permanent metallic implant |
| Drug-eluting bioresorbable scaffold | No permanent implant Superior primary patency compared with angioplasty Restoration of wall vasomotion | Investigational device only Cost analysis pending |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, E.; Varcoe, R.L. Current Status of and Future Prospects for Drug-Eluting Stents and Scaffolds in Infrapopliteal Arteries. J. Clin. Med. 2024, 13, 1757. https://doi.org/10.3390/jcm13061757
Lim E, Varcoe RL. Current Status of and Future Prospects for Drug-Eluting Stents and Scaffolds in Infrapopliteal Arteries. Journal of Clinical Medicine. 2024; 13(6):1757. https://doi.org/10.3390/jcm13061757
Chicago/Turabian StyleLim, Elizabeth, and Ramon L. Varcoe. 2024. "Current Status of and Future Prospects for Drug-Eluting Stents and Scaffolds in Infrapopliteal Arteries" Journal of Clinical Medicine 13, no. 6: 1757. https://doi.org/10.3390/jcm13061757
APA StyleLim, E., & Varcoe, R. L. (2024). Current Status of and Future Prospects for Drug-Eluting Stents and Scaffolds in Infrapopliteal Arteries. Journal of Clinical Medicine, 13(6), 1757. https://doi.org/10.3390/jcm13061757