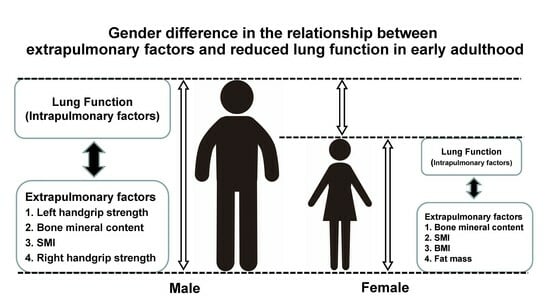
Gender Difference in the Relationship between Extrapulmonary Factors and Reduced Lung Function in Early Adulthood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Assessments
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duong, M.; Islam, S.; Rangarajan, S.; Leong, D.; Kurmi, O.; Teo, K.; Killian, K.; Dagenais, G.; Lear, S.; Wielgosz, A.; et al. Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1 (PURE): An international, community-based cohort study. Lancet Glob. Health 2019, 7, e613–e623. [Google Scholar] [CrossRef]
- Cuttica, M.J.; Colangelo, L.A.; Dransfield, M.T.; Bhatt, S.P.; Rana, J.S.; Jacobs, D.R., Jr.; Thyagarajan, B.; Sidney, S.; Lewis, C.E.; Liu, K.; et al. Lung Function in Young Adults and Risk of Cardiovascular Events Over 29 Years: The CARDIA Study. J. Am. Heart Assoc. 2018, 7, e010672. [Google Scholar] [CrossRef] [PubMed]
- Cuttica, M.J.; Colangelo, L.A.; Shah, S.J.; Lima, J.; Kishi, S.; Arynchyn, A.; Jacobs, D.R., Jr.; Thyagarajan, B.; Liu, K.; Lloyd-Jones, D.; et al. Loss of Lung Health from Young Adulthood and Cardiac Phenotypes in Middle Age. Am. J. Respir. Crit. Care Med. 2015, 192, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.J.; Celli, B.R.; Poblador-Plou, B.; Calderon-Larranaga, A.; de-Torres, J.P.; Gimeno-Feliu, L.A.; Berto, J.; Zulueta, J.J.; Casanova, C.; Pinto-Plata, V.M.; et al. Chronic Obstructive Pulmonary Disease (COPD) as a disease of early aging: Evidence from the EpiChron Cohort. PLoS ONE 2018, 13, e0193143. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.E.; Billheimer, D.; Jenkins, I.C.; Lu, Z.J.; Stern, D.A.; Gerald, L.B.; Carr, T.F.; Guerra, S.; Morgan, W.J.; Wright, A.L.; et al. A Distinct Low Lung Function Trajectory from Childhood to the Fourth Decade of Life. Am. J. Respir. Crit. Care Med. 2016, 194, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Doi, K.; Matsunaga, K.; Takahashi, S.; Donishi, T.; Suga, K.; Oishi, K.; Yasuda, K.; Mimura, Y.; Harada, M.; et al. A Novel Role of Growth Differentiation Factor (GDF)-15 in Overlap with Sedentary Lifestyle and Cognitive Risk in COPD. J. Clin. Med. 2020, 9, 2737. [Google Scholar] [CrossRef]
- Murata, Y.; Hirano, T.; Doi, K.; Fukatsu-Chikumoto, A.; Hamada, K.; Oishi, K.; Kakugawa, T.; Yano, M.; Matsunaga, K. Computed Tomography Lung Density Analysis: An Imaging Biomarker Predicting Physical Inactivity in Chronic Obstructive Pulmonary Disease: A Pilot Study. J. Clin. Med. 2023, 12, 2959. [Google Scholar] [CrossRef]
- Takahashi, S.; Hirano, T.; Yasuda, K.; Donishi, T.; Suga, K.; Doi, K.; Oishi, K.; Ohata, S.; Murata, Y.; Yamaji, Y.; et al. Impact of Frailty on Hippocampal Volume in Patients with Chronic Obstructive Pulmonary Disease. Biomedicines 2021, 9, 1103. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, Y.; Mabu, S.; Hirano, T.; Murata, Y.; Doi, K.; Fukatsu-Chikumoto, A.; Matsunaga, K. Neural Network Approach to Investigating the Importance of Test Items for Predicting Physical Activity in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2023, 12, 4297. [Google Scholar] [CrossRef]
- Matsunaga, K.; Harada, M.; Suizu, J.; Oishi, K.; Asami-Noyama, M.; Hirano, T. Comorbid Conditions in Chronic Obstructive Pulmonary Disease: Potential Therapeutic Targets for Unmet Needs. J. Clin. Med. 2020, 9, 3078. [Google Scholar] [CrossRef]
- GIfCOLDAa. Global Initiative for Chronic Obstructive Lung Disease. Available online: https://goldcopd.org/2020 (accessed on 30 January 2024).
- Lange, P.; Celli, B.; Agusti, A.; Boje Jensen, G.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2015, 373, 111–122. [Google Scholar] [CrossRef]
- Gatt, I.; Smith-Moore, S.; Steggles, C.; Loosemore, M. The Takei Handheld Dynamometer: An Effective Clinical Outcome Measure Tool for Hand and Wrist Function in Boxing. Hand 2018, 13, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Mgbemena, N.C.; Aweto, H.A.; Tella, B.A.; Emeto, T.I.; Malau-Aduli, B.S. Prediction of lung function using handgrip strength in healthy young adults. Physiol. Rep. 2019, 7, e13960. [Google Scholar] [CrossRef]
- Kanai, M.; Kanai, O.; Fujita, K.; Mio, T.; Ito, M. Decreased handgrip strength can predict lung function impairment in male workers: A cross sectional study. BMC Pulm. Med. 2020, 20, 97. [Google Scholar] [CrossRef]
- Sawaya, Y.; Ishizaka, M.; Kubo, A.; Sadakiyo, K.; Yakabi, A.; Sato, T.; Shiba, T.; Onoda, K.; Maruyama, H. Correlation between skeletal muscle mass index and parameters of respiratory function and muscle strength in young healthy adults according to gender. J. Phys. Ther. Sci. 2018, 30, 1424–1427. [Google Scholar] [CrossRef]
- Deodato, M.; Saponaro, S.; Šimunič, B.; Martini, M.; Galmonte, A.; Murena, L.; Buoite Stella, A. Sex-based comparison of trunk flexors and extensors functional and contractile characteristics in young gymnasts. Sport Sci. Health 2023, 20, 147–155. [Google Scholar] [CrossRef]
- Fletcher, C.; Peto, R. The natural history of chronic airflow obstruction. Br. Med. J. 1977, 1, 1645–1648. [Google Scholar] [CrossRef]
- Sears, M.R.; Greene, J.M.; Willan, A.R.; Wiecek, E.M.; Taylor, D.R.; Flannery, E.M.; Cowan, J.O.; Herbison, G.P.; Silva, P.A.; Poulton, R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N. Engl. J. Med. 2003, 349, 1414–1422. [Google Scholar] [CrossRef]
- Barrios, J.; Ai, X. Neurotrophins in Asthma. Curr. Allergy Asthma Rep. 2018, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Kobayashi, H.; Quanjer, P.H.; Omori, H.; Tatsumi, K.; Kanazawa, M.; Clinical Pulmonary Functions Committee of the Japanese Respiratory, S. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir. Investig. 2014, 52, 242–250. [Google Scholar] [CrossRef]
- Keng, B.M.H.; Gao, F.; Teo, L.L.Y.; Lim, W.S.; Tan, R.S.; Ruan, W.; Ewe, S.H.; Koh, W.P.; Koh, A.S. Associations between Skeletal Muscle and Myocardium in Aging: A Syndrome of “Cardio-Sarcopenia”? J. Am. Geriatr. Soc. 2019, 67, 2568–2573. [Google Scholar] [CrossRef]
- Papalexopoulou, N.; Dassios, T.G.; Lunt, A.; Bartlett, F.; Perrin, F.; Bossley, C.J.; Wyatt, H.A.; Greenough, A. Nutritional status and pulmonary outcome in children and young people with cystic fibrosis. Respir. Med. 2018, 142, 60–65. [Google Scholar] [CrossRef]
- Smith, M.P.; Standl, M.; Berdel, D.; von Berg, A.; Bauer, C.P.; Schikowski, T.; Koletzko, S.; Lehmann, I.; Kramer, U.; Heinrich, J.; et al. Handgrip strength is associated with improved spirometry in adolescents. PLoS ONE 2018, 13, e0194560. [Google Scholar] [CrossRef] [PubMed]
- Bellemare, F.; Jeanneret, A.; Couture, J. Sex differences in thoracic dimensions and configuration. Am. J. Respir. Crit. Care Med. 2003, 168, 305–312. [Google Scholar] [CrossRef]
- LoMauro, A.; Aliverti, A. Sex differences in respiratory function. Breathe 2018, 14, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Goodwin, J. Effect of aging on respiratory system physiology and immunology. Clin. Interv. Aging. 2006, 1, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Muscle strength: Clinical and prognostic value of hand-grip dynamometry. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Tak, Y.J.; Ra, Y.; Kim, J.; Han, S.H.; Kim, S.H.; Shin, Y.; Shin, M.J.; Kang, J.H. Reference Respiratory Muscle Strength Values and a Prediction Equation Using Physical Functions for Pulmonary Rehabilitation in Korea. J. Korean Med. Sci. 2023, 38, e325. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, K.; Miao, X.; Wang, J.; Hu, B.; Shen, J.; Hu, X.; Xu, Y.; Yu, B.; Tu, T.; et al. Associations between bone mineral density and chronic obstructive pulmonary disease. J. Int. Med. Res. 2022, 50, 3000605221094644. [Google Scholar] [CrossRef]
- Watanabe, K.; Onoue, A.; Kubota, K.; Higashi, N.; Hayashi, T.; Tsuda, T.; Omori, H. Association between airflow limitation severity and reduced bone mineral density in Japanese men. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
- Sertpoyraz, F.M.; Deniz, S. Bone mineral density and vitamin D levels in patients with group a COPD. Aging Male 2020, 23, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Cvijetic, S.; Pipinic, I.S.; Varnai, V.M.; Macan, J. Relationship between ultrasound bone parameters, lung function, and body mass index in healthy student population. Arh. Hig. Rada Toksikol. 2017, 68, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, D.; Zhao, X.; Chao, L.; Li, Y.; Li, H.; Li, W.; Gui, L.; Wu, W. Association of bone mineral density with lung function in a Chinese general population: The Xinxiang rural cohort study. BMC Pulm. Med. 2019, 19, 239. [Google Scholar] [CrossRef]



| Characteristics | All (n = 151) | Males (n = 86) | Females (n = 65) | p-Value |
|---|---|---|---|---|
| Age (years) | 23.0 (22–24) | 23.0 (22–24) | 23.0 (22–24) | 0.265 |
| Height (cm) | 167 (158–173) | 172 (168–176) | 157 (153–161) | <0.0001 |
| BMI (kg/m2) | 21.0 (19.7–23.0) | 22.1 (20.6–23.9) | 20.0 (18.7–20.9) | <0.0001 |
| SMI (kg/m2) | 7.2 (6.1–7.9) | 7.77 (7.45–8.25) | 5.97 (5.65–6.27) | <0.0001 |
| WBPhA (°), n = 127 | 6.1 (5.3–6.5) | 6.43 (6.02–6.95) | 5.2 (4.9–5.9) | <0.0001 |
| Fat mass (kg), n = 150 | 12.1 (9.6–15.8) | 11.2 (9.6–15.2) | 13 (9.8–16.3) | 0.163 |
| BMC (kg) | 2.63 (2.20–3.07) | 2.99 (2.74–3.28) | 2.15 (1.95–2.3) | <0.0001 |
| Rt HS (kg), n = 139 | 33.5 (26–45) | 44.2 (37.4–47.1) | 25.8 (22.6–28.7) | <0.0001 |
| Lt HS (kg), n = 139 | 31.3 (23.9–41.0) | 40.0 (34.1–44.0) | 23.4 (21.2–25.9) | <0.0001 |
| Birth weight (g), n = 114 | 3020 (2818–3320) | 3100 (3000–3440) | 3000 (2777–3200) | 0.0086 |
| Disease history | ||||
| Asthma | 23 | 14 | 9 | 0.680 |
| Pneumonia | 24 | 12 | 12 | 0.428 |
| Allergic rhinitis | 68 | 36 | 32 | 0.402 |
| Smoking history [Cu/Ex/Non] | 5/6/139 | 5/5/75 | 0/1/64 | 0.0504 |
| VC (L) | 4.05 (3.16–4.73) | 4.69 (4.32–5.12) | 3.12 (2.9–3.34) | <0.0001 |
| FVC (L) | 4.17 (3.25–4.72) | 4.63 (4.32–5.16) | 3.15 (2.89–3.46) | <0.0001 |
| FEV1 (L) | 3.62 (2.9–4.22) | 4.13 (3.72–4.49) | 2.86 (2.64–3.14) | <0.0001 |
| FEV1/FVC (%) | 89.3 (85.1–93.4) | 89.1 (83.9–92.3) | 90.9 (87.1–94.5) | 0.0509 |
| %VC (%) | 95.5 (87.9–103.4) | 96.4 (89.7–104.7) | 93.3 (86.5–102.9) | 0.160 |
| % FVC (%) | 97.1 (89.3–104.9) | 97.6 (90.5–106.0) | 96.8 (88.2–104.3) | 0.399 |
| %FEV1 (%) | 98.1 (90.9–105.9) | 99.0 (90.9–106.5) | 97.1 (90.7–104.6) | 0.456 |
| VC< LLN, n (%) | 19 (12.6) | 8 (9.3) | 11 (16.9) | 0.162 |
| FVC< LLN, n (%) | 15 (9.9) | 8 (9.3) | 7 (10.8) | 0.765 |
| FEV1 < LLN, n (%) | 15 (9.9) | 6 (7.0) | 9 (13.8) | 0.162 |
| FEV1/FVC< LLN, n (%) | 11 (7.3) | 3 (3.5) | 8 (12.3) | 0.0389 |
| FVC | FEV1 | |||
|---|---|---|---|---|
| ρ | p-Value | ρ | p-Value | |
| BMI | 0.447 | <0.0001 | 0.389 | <0.0001 |
| SMI | 0.828 | <0.0001 | 0.788 | <0.0001 |
| WBPhA | 0.523 | <0.0001 | 0.498 | <0.0001 |
| Fat mass | −0.07 | 0.4148 | −0.11 | 0.1647 |
| BMC | 0.840 | <0.0001 | 0.818 | <0.0001 |
| Rt HS | 0.756 | <0.0001 | 0.740 | <0.0001 |
| Lt HS | 0.834 | <0.0001 | 0.807 | <0.0001 |
| Birth weight | 0.246 | 0.0083 | 0.265 | 0.0043 |
| Males | Females | ||||
|---|---|---|---|---|---|
| ρ | p-Value | ρ | p-Value | ||
| Lt HS | 0.471 | <0.0001 | BMC | 0.477 | <0.0001 |
| BMC | 0.463 | <0.0001 | SMI | 0.384 | 0.002 |
| SMI | 0.276 | 0.010 | BMI | 0.261 | 0.036 |
| Rt HS | 0.240 | 0.036 | Fat mass | 0.246 | 0.048 |
| Birth weight | 0.235 | 0.076 | Lt HS | 0.212 | 0.098 |
| WBPhA | 0.069 | 0.562 | Rt HS | 0.132 | 0.307 |
| Fat mass | −0.198 | 0.069 | WBPhA | −0.111 | 0.423 |
| BMI | −0.122 | 0.265 | Birth weight | −0.062 | 0.647 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doi, K.; Hirano, T.; Oishi, K.; Fukatsu-Chikumoto, A.; Ohteru, Y.; Hamada, K.; Ohata, S.; Murata, Y.; Yamaji, Y.; Asami-Noyama, M.; et al. Gender Difference in the Relationship between Extrapulmonary Factors and Reduced Lung Function in Early Adulthood. J. Clin. Med. 2024, 13, 1769. https://doi.org/10.3390/jcm13061769
Doi K, Hirano T, Oishi K, Fukatsu-Chikumoto A, Ohteru Y, Hamada K, Ohata S, Murata Y, Yamaji Y, Asami-Noyama M, et al. Gender Difference in the Relationship between Extrapulmonary Factors and Reduced Lung Function in Early Adulthood. Journal of Clinical Medicine. 2024; 13(6):1769. https://doi.org/10.3390/jcm13061769
Chicago/Turabian StyleDoi, Keiko, Tsunahiko Hirano, Keiji Oishi, Ayumi Fukatsu-Chikumoto, Yuichi Ohteru, Kazuki Hamada, Shuichiro Ohata, Yoriyuki Murata, Yoshikazu Yamaji, Maki Asami-Noyama, and et al. 2024. "Gender Difference in the Relationship between Extrapulmonary Factors and Reduced Lung Function in Early Adulthood" Journal of Clinical Medicine 13, no. 6: 1769. https://doi.org/10.3390/jcm13061769
APA StyleDoi, K., Hirano, T., Oishi, K., Fukatsu-Chikumoto, A., Ohteru, Y., Hamada, K., Ohata, S., Murata, Y., Yamaji, Y., Asami-Noyama, M., Edakuni, N., Kakugawa, T., & Matsunaga, K. (2024). Gender Difference in the Relationship between Extrapulmonary Factors and Reduced Lung Function in Early Adulthood. Journal of Clinical Medicine, 13(6), 1769. https://doi.org/10.3390/jcm13061769