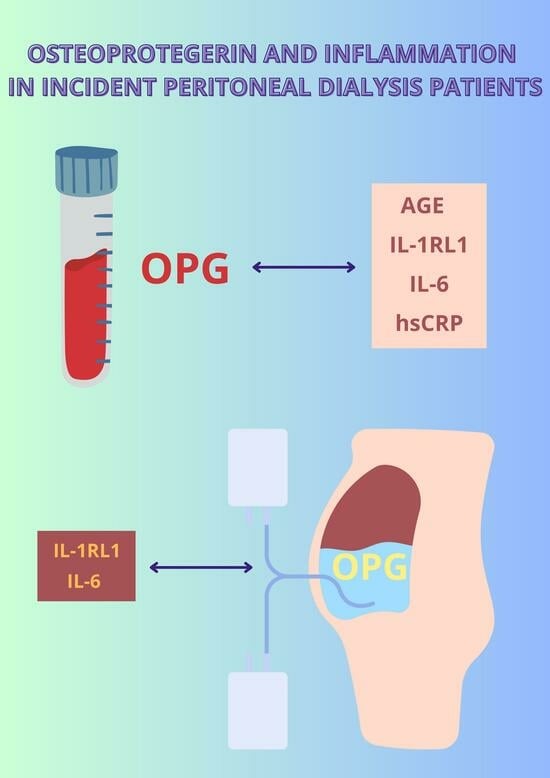
Osteoprotegerin and Inflammation in Incident Peritoneal Dialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Data Collection
2.4. Procedure
2.5. Statistical Analysis
3. Results
3.1. Relationship between OPG and Clinical Features
3.2. Relationship between OPG and Proinflammatory Mediators’ Concentrations in Plasma and Dialysate
3.3. Multivariate Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.M.; Kim, S.H.; Yoon, H.J. Prediction of incident chronic kidney disease in a population with normal renal function and normo-proteinuria. PLoS ONE 2023, 18, e0285102. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E. Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014, 85, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Rodríguez, E.; Pizarro-Sánchez, S.; Sanz, A.B.; Ramos, A.M.; Sanchez-Niño, M.D.; Martin-Cleary, C.; Fernandez-Fernandez, B.; Ortiz, A. Inflammatory cytokines as uremic toxins: “ni son todos los que estan, ni estan todos los que son”. Toxins 2017, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transplant. 2018, 33, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Lei, C.T.; Zhang, C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front. Immunol. 2017, 8, 255909. [Google Scholar] [CrossRef] [PubMed]
- Gungor, O.; Unal, H.U.; Guclu, A.; Gezer, M.; Eyileten, T.; Guzel, F.B.; Altunoren, O.; Erken, E.; Oguz, Y.; Kocyigit, I.; et al. IL-33 and ST2 levels in chronic kidney disease: Associations with inflammation, vascular abnormalities, cardiovascular events, and survival. PLoS ONE 2017, 12, e0178939. [Google Scholar] [CrossRef]
- Hansson, J.H.; Watnick, S. Update on Peritoneal Dialysis: Core Curriculum 2016. Am. J. Kidney Dis. 2016, 67, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Lambie, M.; Chess, J.; Donovan, K.L.; Kim, Y.L.; Do, J.Y.; Lee, H.B.; Noh, H.; Williams, P.F.; Williams, A.J.; Davison, S.; et al. Independent effects of systemic and peritoneal inflammation on peritoneal dialysis survival. J. Am. Soc. Nephrol. 2013, 24, 2071–2080. [Google Scholar] [CrossRef]
- Williams, J.D.; Craig, K.J.; Topley, N.; Von Ruhland, C.; Fallon, M.; Newman, G.R.; Mackenzie, R.K.; Williams, G.T. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 2002, 13, 470–479. [Google Scholar] [CrossRef]
- Tawada, M.; Hamada, C.; Suzuki, Y.; Sakata, F.; Sun, T.; Kinashi, H.; Katsuno, T.; Takei, Y.; Maruyama, S.; Honda, K.; et al. Effects of long-term treatment with low-GDP, pH-neutral solutions on peritoneal membranes in peritoneal dialysis patients. Clin. Exp. Nephrol. 2019, 23, 689–699. [Google Scholar] [CrossRef]
- Schneeweis, L.A.; Willard, D.; Milla, M.E. Functional dissection of osteoprotegerin and its interaction with receptor activator of NF-κB ligand. J. Biol. Chem. 2005, 280, 41155–41164. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N. Bone and the Immune System. Toxicol. Pathol. 2017, 45, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, M.; Koepp, R.; Cortis, J.; Komrakova, M.; Schieker, M.; Hempel, U.; Siggelkow, H. IL-6, IL-1β, and TNF-α only in combination influence the osteoporotic phenotype in Crohn’s patients via bone formation and bone resorption. Adv. Clin. Exp. Med. 2018, 27, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The role of osteoprotegerin and its ligands in vascular function. Int. J. Mol. Sci. 2019, 20, 705. [Google Scholar] [CrossRef] [PubMed]
- Janda, K.; Krzanowski, M.; Chowaniec, E.; Kuśnierz-Cabala, B.; Dumnicka, P.; Kraśniak, A.; Podolec, P.; Sułowicz, W. Osteoprotegerin as a marker of cardiovascular risk in patients on peritoneal dialysis. Pol. Arch. Med. Wewn. 2013, 123, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Krzanowski, M.; Janda, K.; Dumnicka, P.; Stompór, M.; Kuśnierz-Cabala, B.; Grodzicki, T.; Sułowicz, W. Relationship between aortic pulse wave velocity, selected proinflammatory cytokines, and vascular calcification parameters in peritoneal dialysis patients. J. Hypertens. 2014, 32, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Q.; Lin, S.; Xu, P.C.; Zheng, Z.F.; Jia, J.Y. Serum osteoprotegerin measurement for early diagnosis of chronic kidney disease-mineral and bone disorder. Nephrology 2011, 16, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Toffoli, B.; Bossi, F.; Candido, R.; Stenner, E.; Carretta, R.; Barbone, F.; Fabris, B. Circulating osteoprotegerin is associated with chronic kidney disease in hypertensive patients. BMC Nephrol. 2017, 18, 219. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.M.; Tarnow, L.; Hansen, T.K.; Parving, H.H.; Flyvbjerg, A. Plasma osteoprotegerin levels are associated with glycaemic status, systolic blood pressure, kidney function and cardiovascular mordibity in type 1 diabetic patients. Eur. J. Endocrinol. 2006, 154, 75–81. [Google Scholar] [CrossRef]
- Ozkok, A.; Caliskan, Y.; Sakaci, T.; Erten, G.; Karahan, G.; Ozel, A.; Unsal, A.; Yildiz, A. Osteoprotegerin/RANKL axis and progression of coronary artery calcification in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2012, 7, 965–973. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Hayashi, S.Y.; Riella, M.C.; Lindholm, B. Elevated levels of plasma osteoprotegerin are associated with all-cause mortality risk and atherosclerosis in patients with stages 3 to 5 chronic kidney disease. Braz. J. Med. Biol. Res. 2014, 47, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Krzanowski, M.; Krzanowska, K.; Dumnicka, P.; Gajda, M.; Woziwodzka, K.; Fedak, D.; Grodzicki, T.; Litwin, J.A.; Sułowicz, W. Elevated Circulating Osteoprotegerin Levels in the Plasma of Hemodialyzed Patients With Severe Artery Calcification. Ther. Apher. Dial. 2018, 22, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Kuźniewski, M.; Fedak, D.; Dumnicka, P.; Stępień, E.; Kuśnierz-Cabala, B.; Cwynar, M.; Sułowicz, W. Osteoprotegerin and osteoprotegerin/TRAIL ratio are associated with cardiovascular dysfunction and mortality among patients with renal failure. Adv. Med. Sci. 2016, 61, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, J.; Stopiński, M.; Mucha, K.; Pac, M.; Gołębiowski, M.; A Niewczas, M.; Pączek, L.; Foroncewicz, B. Circulating osteoprotegerin in chronic kidney disease and all-cause mortality. Int. J. Gen. Med. 2021, 14, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Ruggenenti, P.; Schieppati, A.; Remuzzi, G. Progression, remission, regression of chronic renal diseases. Lancet 2001, 357, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Dahle, D.O.; Mjøen, G.; Weihrauch, G.; Scharnagl, H.; Dobnig, H.; März, W.; Jardine, A.; Fellström, B.; Holdaas, H. Osteoprotegerin as a predictor of renal and cardiovascular outcomes in renal transplant recipients: Follow-up data from the ALERT study. Nephrol. Dial. Transplant. 2012, 27, 2571–2575. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Struyf, S.; Mohammad, G.; Gouwy, M.; Rytinx, P.; Siddiquei, M.M.; Hernández, C.; Alam, K.; Mousa, A.; De Hertogh, G.; et al. Osteoprotegerin is a new regulator of inflammation and angiogenesis in proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3189–3201. [Google Scholar] [CrossRef]
- Benito-Martin, A.; Ucero, A.C.; Zubiri, I.; Posada-Ayala, M.; Fernandez-Fernandez, B.; Cannata-Ortiz, P.; Sanchez-Nino, M.D.; Ruiz-Ortega, M.; Egido, J.; Alvarez-Llamas, G.; et al. Osteoprotegerin in Exosome-Like Vesicles from Human Cultured Tubular Cells and Urine. PLoS ONE 2013, 8, e72387. [Google Scholar]
- Ávila, M.; Prado, M.D.C.; Romero, R.; Córdova, R.; Rigo, M.d.C.; Trejo, M.; Mora, C.; Paniagua, R. Osteoprotegerin Is a Better Predictor for Cardiovascular and All-Cause Mortality than Vascular Calcifications in a Multicenter Cohort of Patients on Peritoneal Dialysis. Biomolecules 2022, 12, 551. [Google Scholar] [CrossRef]
- Knudsen, L.S.; Christensen, I.J.; Lottenburger, T.; Svendsen, M.N.; Nielsen, H.J.; Nielsen, L.; Hørslev-Petersen, K.; Jensen, J.E.B.; Kollerup, G.; Johansen, J.S. Pre-analytical and biological variability in circulating interleukin 6 in healthy subjects and patients with rheumatoid arthritis. Biomarkers 2008, 13, 59–78. [Google Scholar] [CrossRef]
- Vik, A.; Brodin, E.E.; Mathiesen, E.B.; Brox, J.; Jørgensen, L.; Njølstad, I.; Brækkan, S.K.; Hansen, J.-B. Serum osteoprotegerin and renal function in the general population: The Tromsø Study. Clin. Kidney J. 2017, 10, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.M.; Do, H.M.; Kim, E.J.; Lee, M.J.; Shin, D.H.; Kim, S.J.; Oh, H.J.; Yoo, D.E.; Kim, J.-K.; Park, J.T.; et al. Elevated osteoprotegerin is associated with inflammation, malnutrition and new onset cardiovascular events in peritoneal dialysis patients. Atherosclerosis 2011, 219, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Y.; Jing, H.Y.; Ma, Y.R. Interleukin-33/ Suppression of Tumorigenicity 2 in Renal Fibrosis: Emerging Roles in Prognosis and Treatment. Front. Physiol. 2022, 12, 792897. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Yu, H.; Ma, X.; Zhang, C.; Qu, B.; Zhang, W. Superior prognostic value of soluble suppression of tumorigenicity 2 for the short-term mortality of maintenance hemodialysis patients compared with NT-proBNP: A prospective cohort study. Ren. Fail. 2020, 42, 523–530. [Google Scholar] [CrossRef]
- Choi, Y.B.; Lee, M.J.; Park, J.T.; Han, S.H.; Kang, S.-W.; Yoo, T.-H.; Kim, H.J. Prognostic value of soluble ST2 and soluble LR11 on mortality and cardiovascular events in peritoneal dialysis patients. BMC Nephrol. 2020, 21, 228. [Google Scholar] [CrossRef]
- Guo, G.; Huang, A.; Huang, X.; Xu, T.; Yao, L. Prognostic Value of Soluble Suppression of Tumorigenicity 2 in Chronic Kidney Disease Patients: A Meta-Analysis. Dis. Markers 2021, 2021, 8881393. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, K.H.; Lee, S.; Jo, J.; Park, J.Y.; Park, M.; Tsogbadrakh, B.; Lee, J.P.; Lee, J.W.; Kim, D.K.; et al. ST2 blockade mitigates peritoneal fibrosis induced by TGF-β and high glucose. J. Cell Mol. Med. 2019, 23, 6872–6884. [Google Scholar] [CrossRef]



| Mean ± SD | Median | IQR | |
|---|---|---|---|
| Age (years) | 50.35 ± 13.82 | ||
| BMI (kg/m2) | 24.41 ± 3.72 | ||
| BSA (m2) | 1.75 ± 0.16 | ||
| Hemoglobin (mmol/L) | 7.11 ± 0.71 | ||
| Hematocrit (%) | 34.62 ± 3.31 | ||
| Mean corpuscular volume (fL) | 88.10 | 4.90 | |
| White blood cell count (G/L) | 6.93 | 3.01 | |
| Urea (mg/dL) | 90.19 ± 30.74 | ||
| Creatinine (mg/dL) | 4.10 | 2.87 | |
| Uric acid (mg/dL) | 5.64 ± 1.22 | ||
| Sodium (mmol/L) | 139.86 ± 3.29 | ||
| Potassium (mmol/L) | 4.11 ± 0.45 | ||
| Total calcium (mmol/L) | 2.29 ± 0.19 | ||
| Phosphate (mmol/L) | 1.34 | 0.34 | |
| Parathyroid hormone (pg/mL) | 212.80 | 207.5 | |
| FGF 23 (pg/mL) | 30.65 ± 2.74 | ||
| Sclerostin (ng/mL) | 1.82 | 1.76 | |
| Total protein (g/L) | 63.24 ± 6.08 | ||
| Albumin (g/L) | 36.10 ± 3.86 | ||
| Alkaline phosphatase (U/L) | 81.00 | 36.00 | |
| Total bilirubin (mg/dL) | 0.40 | 0.22 | |
| Total cholesterol (mg/dL) | 213.00 | 72.00 | |
| LDL cholesterol (mg/dL) | 129.00 | 61.00 | |
| HDL cholesterol (mg/dL) | 56.05 ± 14.67 | ||
| Triglycerides (mg/dL) | 127.00 | 44.00 | |
| Glucose (mg/dL) | 95.00 | 50.00 | |
| Residual diuresis (mL) | 1848.91 ± 936.24 | ||
| Renal Kt/V | 1.45 | 1.31 | |
| Dialysate Kt/V | 1.42 ± 0.43 | ||
| Total Kt/V | 2.93 | 1.50 | |
| UF (mL) | 645.40 ± 274.83 | ||
| PET D/P creatinine | 0.69 ± 0.08 |
| Parameter (Unit) | Mean ± SD | Median | IQR |
|---|---|---|---|
| Plasma IL-1RL1 (sST2) (pg/mL) | 3583.25 | 1255.70 | |
| Dialysate IL-1RL1 (sST2) (pg/mL) | 432.08 | 210.59 | |
| Plasma IL-33 (pg/mL) | 73.14 | 14.62 | |
| Dialysate IL-33 (pg/mL) | 9.09 | 2.18 | |
| Plasma IL-6 (pg/mL) | 4.60 | 4.00 | |
| Dialysate IL-6 (pg/mL) | 26.00 | 32.70 | |
| Plasma hsCRP (mg/L) | 2.64 | 7.22 | |
| Dialysate hsCRP (mg/L) | 0.12 | 0.06 | |
| Plasma OPG (pmol/L) | 15.50 ± 3.62 | ||
| Dialysate OPG (pmol/L) | 2.81 | 1.64 |
| Plasma OPG | |||
|---|---|---|---|
| Independent variables | Beta (ß) | Beta (ß) SE | p value |
| age | 0.36 | 0.13 | 0.01 |
| log_plasma hsCRP | 0.32 | 0.13 | 0.02 |
| log_plasma IL-1RL1 (sST2) | 0.38 | 0.13 | 0.006 |
| Plasma OPG | |||
|---|---|---|---|
| Independent variables | Beta (ß) | Beta (ß) SE | p value |
| age | 0.30 | 0.15 | 0.05 |
| log_plasma IL-6 | 0.32 | 0.15 | 0.04 |
| log_plasma IL-1RL1 (sST2) | 0.35 | 0.13 | 0.01 |
| Dialysate OPG | |||
|---|---|---|---|
| Independent variables | Beta (ß) | Beta (ß) SE | p value |
| log_dialysate IL-6 | 0.37 | 0.15 | 0.02 |
| log_dialysate IL1RL1 (sST2) | 0.27 | 0.15 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małecki, M.; Okulewicz, P.; Lisak, M.; Safranow, K.; Domański, L.; Ciechanowski, K.; Gołembiewska, E. Osteoprotegerin and Inflammation in Incident Peritoneal Dialysis Patients. J. Clin. Med. 2024, 13, 2345. https://doi.org/10.3390/jcm13082345
Małecki M, Okulewicz P, Lisak M, Safranow K, Domański L, Ciechanowski K, Gołembiewska E. Osteoprotegerin and Inflammation in Incident Peritoneal Dialysis Patients. Journal of Clinical Medicine. 2024; 13(8):2345. https://doi.org/10.3390/jcm13082345
Chicago/Turabian StyleMałecki, Michał, Patrycja Okulewicz, Marcin Lisak, Krzysztof Safranow, Leszek Domański, Kazimierz Ciechanowski, and Edyta Gołembiewska. 2024. "Osteoprotegerin and Inflammation in Incident Peritoneal Dialysis Patients" Journal of Clinical Medicine 13, no. 8: 2345. https://doi.org/10.3390/jcm13082345
APA StyleMałecki, M., Okulewicz, P., Lisak, M., Safranow, K., Domański, L., Ciechanowski, K., & Gołembiewska, E. (2024). Osteoprotegerin and Inflammation in Incident Peritoneal Dialysis Patients. Journal of Clinical Medicine, 13(8), 2345. https://doi.org/10.3390/jcm13082345