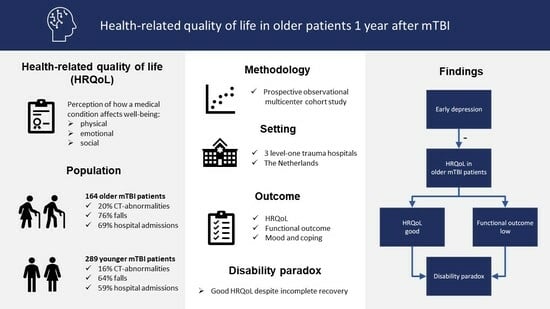
Good Health-Related Quality of Life in Older Patients One Year after mTBI despite Incomplete Recovery: An Indication of the Disability Paradox?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Measures
2.2.1. Data Obtained at Two Weeks
2.2.2. Data Obtained at 12 Months
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Post-traumatic Complaints, Emotional Distress and Coping at 2 Weeks Post-Injury
3.3. Health-Related Quality of Life and Functional Recovery
3.4. Predictors of One-Year Post-Injury HRQoL for Older and Younger Age Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Belli, A.; Bragge, P.; Brazinova, A.; Buki, A.; Chesnut, R.M.; Citerio, G.; et al. InTBIR Participants and Investigators Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [PubMed]
- Scholten, A.C.; Haagsma, J.A.; Panneman, M.J.; van Beeck, E.F.; Polinder, S. Traumatic brain injury in the Netherlands: Incidence, costs and disability-adjusted life years. PLoS ONE 2014, 9, e110905. [Google Scholar] [CrossRef] [PubMed]
- Levin, H.S.; Diaz-Arrastia, R.R. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2015, 14, 506–517. [Google Scholar] [CrossRef]
- Tillou, A.; Kelley-Quon, L.; Burruss, S.; Morley, E.; Cryer, H.; Cohen, M.; Min, L. Long-term postinjury functional recovery: Outcomes of geriatric consultation. JAMA Surg. 2014, 149, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Roozenbeek, B.; Maas, A.I.; Menon, D.K. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 2013, 9, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Brazinova, A.; Rehorcikova, V.; Taylor, M.S.; Buckova, V.; Majdan, M.; Psota, M.; Peeters, W.; Feigin, V.; Theadom, A.; Holkovic, L.; et al. Epidemiology of Traumatic Brain Injury in Europe: A Living Systematic Review. J. Neurotrauma 2021, 38, 1411–1440. [Google Scholar] [CrossRef] [PubMed]
- Dams-O’Connor, K.; Gibbons, L.E.; Landau, A.; Larson, E.B.; Crane, P.K. Health Problems Precede Traumatic Brain Injury in Older Adults. J. Am. Geriatr. Soc. 2016, 64, 844–848. [Google Scholar] [CrossRef]
- Evans, D.C.; Cook, C.H.; Christy, J.M.; Murphy, C.V.; Gerlach, A.T.; Eiferman, D.; Lindsey, D.E.; Whitmill, M.L.; Papadimos, T.J.; Beery, P.R.; et al. Comorbidity-polypharmacy scoring facilitates outcome prediction in older trauma patients. J. Am. Geriatr. Soc. 2012, 60, 1465–1470. [Google Scholar] [CrossRef]
- Evans, D.C.; Gerlach, A.T.; Christy, J.M.; Jarvis, A.M.; Lindsey, D.E.; Whitmill, M.L.; Eiferman, D.; Murphy, C.V.; Cook, C.H.; Beery, P.R.; et al. Pre-injury polypharmacy as a predictor of outcomes in trauma patients. Int. J. Crit. Illn. Inj. Sci. 2011, 1, 104–109. [Google Scholar] [CrossRef]
- Gardner, R.C.; Dams-O’Connor, K.; Morrissey, M.R.; Manley, G.T. Geriatric Traumatic Brain Injury: Epidemiology, Outcomes, Knowledge Gaps, and Future Directions. J. Neurotrauma 2018, 35, 889–906. [Google Scholar] [CrossRef]
- Laic, R.A.G.; Bogaert, L.; Vander Sloten, J.; Depreitere, B. Functional outcome, dependency and well-being after traumatic brain injury in the elderly population: A systematic review and meta-analysis. Brain Spine 2021, 1, 100849. [Google Scholar] [CrossRef]
- Thompson, H.J.; McCormick, W.C.; Kagan, S.H. Traumatic brain injury in older adults: Epidemiology, outcomes, and future implications. J. Am. Geriatr. Soc. 2006, 54, 1590–1595. [Google Scholar] [CrossRef]
- af Geijerstam, J.L.; Britton, M. Mild head injury—Mortality and complication rate: Meta-analysis of findings in a systematic literature review. Acta Neurochir. (Wien) 2003, 145, 843–850; discussion 850. [Google Scholar] [CrossRef]
- Harvey, L.A.; Close, J.C. Traumatic brain injury in older adults: Characteristics, causes and consequences. Injury 2012, 43, 1821–1826. [Google Scholar] [CrossRef]
- Susman, M.; DiRusso, S.M.; Sullivan, T.; Risucci, D.; Nealon, P.; Cuff, S.; Haider, A.; Benzil, D. Traumatic brain injury in the elderly: Increased mortality and worse functional outcome at discharge despite lower injury severity. J. Trauma 2002, 53, 219–223; discussion 223–224. [Google Scholar] [CrossRef]
- Kumar, R.G.; Juengst, S.B.; Wang, Z.; Dams-O’Connor, K.; Dikmen, S.S.; O’Neil-Pirozzi, T.M.; Dahdah, M.N.; Hammond, F.M.; Felix, E.R.; Arenth, P.M.; et al. Epidemiology of Comorbid Conditions among Adults 50 Years and Older with Traumatic Brain Injury. J. Head Trauma Rehabil. 2018, 33, 15–24. [Google Scholar] [CrossRef]
- Karimi, M.; Brazier, J. Health, Health-Related Quality of Life, and Quality of Life: What is the Difference? Pharmacoeconomics 2016, 34, 645–649. [Google Scholar] [CrossRef]
- Wilson, J.T.; Pettigrew, L.E.; Teasdale, G.M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. J. Neurotrauma 1998, 15, 573–585. [Google Scholar] [CrossRef]
- Voormolen, D.C.; Polinder, S.; von Steinbuechel, N.; Vos, P.E.; Cnossen, M.C.; Haagsma, J.A. The association between post-concussion symptoms and health-related quality of life in patients with mild traumatic brain injury. Injury 2019, 50, 1068–1074. [Google Scholar] [CrossRef]
- Lin, M.R.; Chiu, W.T.; Chen, Y.J.; Yu, W.Y.; Huang, S.J.; Tsai, M.D. Longitudinal changes in the health-related quality of life during the first year after traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 474–480. [Google Scholar] [CrossRef]
- Haagsma, J.A.; Polinder, S.; Olff, M.; Toet, H.; Bonsel, G.J.; van Beeck, E.F. Posttraumatic stress symptoms and health-related quality of life: A two year follow up study of injury treated at the emergency department. BMC Psychiatry 2012, 12, 1. [Google Scholar] [CrossRef]
- Kristman, V.L.; Brison, R.J.; Bedard, M.; Reguly, P.; Chisholm, S. Prognostic Markers for Poor Recovery After Mild Traumatic Brain Injury in Older Adults: A Pilot Cohort Study. J. Head Trauma Rehabil. 2016, 31, E33–E43. [Google Scholar] [CrossRef]
- van der Naalt, J.; Timmerman, M.E.; de Koning, M.E.; van der Horn, H.J.; Scheenen, M.E.; Jacobs, B.; Hageman, G.; Yilmaz, T.; Roks, G.; Spikman, J.M. Early predictors of outcome after mild traumatic brain injury (UPFRONT): An observational cohort study. Lancet Neurol. 2017, 16, 532–540. [Google Scholar] [CrossRef]
- Haydel, M.J.; Preston, C.A.; Mills, T.J.; Luber, S.; Blaudeau, E.; DeBlieux, P.M. Indications for computed tomography in patients with minor head injury. N. Engl. J. Med. 2000, 343, 100–105. [Google Scholar] [CrossRef]
- Smits, M.; Dippel, D.W.; Steyerberg, E.W.; de Haan, G.G.; Dekker, H.M.; Vos, P.E.; Kool, D.R.; Nederkoorn, P.J.; Hofman, P.A.; Twijnstra, A.; et al. Predicting intracranial traumatic findings on computed tomography in patients with minor head injury: The CHIP prediction rule. Ann. Intern. Med. 2007, 146, 397–405. [Google Scholar] [CrossRef]
- Coffeng, S.M.; Foks, K.A.; van den Brand Crispijn, L.; Jellema, K.; Dippel, D.W.J.; Jacobs, B.; van der Naalt, J. Evaluation of Clinical Characteristics and CT Decision Rules in Elderly Patients with Minor Head Injury: A Prospective Multicenter Cohort Study. J. Clin. Med. 2023, 12, 982. [Google Scholar] [CrossRef]
- Westcott, M.C.; Alfano, D.P. The symptom checklist-90-revised and mild traumatic brain injury. Brain Inj. 2005, 19, 1261–1267. [Google Scholar] [CrossRef]
- de Koning, M.E.; Gareb, B.; El Moumni, M.; Scheenen, M.E.; van der Horn, H.J.; Timmerman, M.E.; Spikman, J.M.; van der Naalt, J. Subacute posttraumatic complaints and psychological distress in trauma patients with or without mild traumatic brain injury. Injury 2016, 47, 2041–2047. [Google Scholar] [CrossRef]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Horowitz, M.; Wilner, N.; Alvarez, W. Impact of Event Scale: A measure of subjective stress. Psychosom. Med. 1979, 41, 209–218. [Google Scholar] [CrossRef]
- Curran, C.A.; Ponsford, J.L.; Crowe, S. Coping strategies and emotional outcome following traumatic brain injury: A comparison with orthopedic patients. J. Head Trauma Rehabil. 2000, 15, 1256–1274. [Google Scholar] [CrossRef]
- Skevington, S.M.; Lotfy, M.; O’Connell, K.A.; WHOQOL Group. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual. Life Res. 2004, 13, 299–310. [Google Scholar] [CrossRef]
- De Vries, J.; Van Heck, G.L. The World Health Organization Quality of Life assessment instrument (WHOQOL-100): Validation study with the dutch version. Eur. J. Psychol. Assess. 1997, 13, 164–178. [Google Scholar] [CrossRef]
- De Vries, J.; Den Oudsten, B.L. Manual WHOQOL-100 and WHOQOL-BREF, Revised version; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Weber, K.T.; Guimaraes, V.A.; Pontes Neto, O.M.; Leite, J.P.; Takayanagui, O.M.; Santos-Pontelli, T.E. Predictors of quality of life after moderate to severe traumatic brain injury. Arq. Neuropsiquiatr. 2016, 74, 409–415. [Google Scholar] [CrossRef]
- Scholten, A.C.; Haagsma, J.A.; Andriessen, T.M.; Vos, P.E.; Steyerberg, E.W.; van Beeck, E.F.; Polinder, S. Health-related quality of life after mild, moderate and severe traumatic brain injury: Patterns and predictors of suboptimal functioning during the first year after injury. Injury 2015, 46, 616–624. [Google Scholar] [CrossRef]
- Yousefzadeh-Chabok, S.; Kapourchali, F.R.; Ramezani, S. Determinants of long-term health-related quality of life in adult patients with mild traumatic brain injury. Eur. J. Trauma Emerg. Surg. 2021, 47, 839–846. [Google Scholar] [CrossRef]
- Hume, C.H.; Mitra, B.; Wright, B.J.; Kinsella, G.J. Quality of life and psychological health after mild traumatic brain injury in older people: Three- and six-month follow up. Brain Inj. 2023, 37, 1262–1271. [Google Scholar] [CrossRef]
- van der Vlegel, M.; Mikolic, A.; Lee Hee, Q.; Kaplan, Z.L.R.; Retel Helmrich Isabel, R.A.; van Veen, E.; Andelic, N.; Steinbuechel, N.V.; Plass, A.M.; Zeldovich, M.; et al. CENTER-TBI Participants and Investigators Health care utilization and outcomes in older adults after Traumatic Brain Injury: A CENTER-TBI study. Injury 2022, 53, 2774–2782. [Google Scholar] [CrossRef]
- Retel Helmrich, I.R.A.; van Klaveren, D.; Andelic, N.; Lingsma, H.; Maas, A.; Menon, D.; Polinder, S.; Roe, C.; Steyerberg, E.W.; Van Veen, E.; et al. Discrepancy between disability and reported well-being after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2022, 93, 785–796. [Google Scholar] [CrossRef]
- Nichol, A.D.; Higgins, A.M.; Gabbe, B.J.; Murray, L.J.; Cooper, D.J.; Cameron, P.A. Measuring functional and quality of life outcomes following major head injury: Common scales and checklists. Injury 2011, 42, 281–287. [Google Scholar] [CrossRef]
- Haagsma, J.A.; Scholten, A.C.; Andriessen, T.M.; Vos, P.E.; Van Beeck, E.F.; Polinder, S. Impact of depression and post-traumatic stress disorder on functional outcome and health-related quality of life of patients with mild traumatic brain injury. J. Neurotrauma 2015, 32, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Moss-Morris, R.; Peveler, R.; Mogg, K.; Bradley, B.P.; Belli, A. When a minor head injury results in enduring symptoms: A prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2012, 83, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Popov, N.; Mercier, L.J.; King, R.; Fung, T.; Debert, C.T. Factors Associated with Quality of Life in Adults with Persistent Post-Concussion Symptoms. Can. J. Neurol. Sci. 2022, 49, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Juengst, S.B.; Kumar, R.G.; Wagner, A.K. A narrative literature review of depression following traumatic brain injury: Prevalence, impact, and management challenges. Psychol. Res. Behav. Manag. 2017, 10, 175–186. [Google Scholar] [CrossRef] [PubMed]
- von Brevern, M.; Radtke, A.; Lezius, F.; Feldmann, M.; Ziese, T.; Lempert, T.; Neuhauser, H. Epidemiology of benign paroxysmal positional vertigo: A population based study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Tjell, C.; Iglebekk, W.; Borenstein, P. Can a Chronic BPPV With a History of Trauma be the Trigger of Symptoms in Vestibular Migraine, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), and Whiplash Associated Disorders (WAD)? A Retrospective Cohort Study. Otol. Neurotol. 2019, 40, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Van De Wyngaerde Kelly, M.; Lee, M.K.; Jacobson, G.P.; Pasupathy, K.; Romero-Brufau, S.; McCaslin, D.L. The Component Structure of the Dizziness Handicap Inventory (DHI): A Reappraisal. Otol. Neurotol. 2019, 40, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Bombardier, C.H.; Fann, J.R.; Temkin, N.R.; Esselman, P.C.; Barber, J.; Dikmen, S.S. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 2010, 303, 1938–1945. [Google Scholar] [CrossRef]
- Vikane, E.; Hellstrom, T.; Roe, C.; Bautz-Holter, E.; Assmus, J.; Skouen, J.S. Missing a follow-up after mild traumatic brain injury—Does it matter? Brain Inj. 2014, 28, 1374–1380. [Google Scholar] [CrossRef]
- Corrigan, J.D.; Harrison-Felix, C.; Bogner, J.; Dijkers, M.; Terrill, M.S.; Whiteneck, G. Systematic bias in traumatic brain injury outcome studies because of loss to follow-up. Arch. Phys. Med. Rehabil. 2003, 84, 153–160. [Google Scholar] [CrossRef]
- McMahon, P.; Hricik, A.; Yue, J.K.; Puccio, A.M.; Inoue, T.; Lingsma, H.F.; Beers, S.R.; Gordon, W.A.; Valadka, A.B.; Manley, G.T.; et al. TRACK-TBI Investigators Symptomatology and functional outcome in mild traumatic brain injury: Results from the prospective TRACK-TBI study. J. Neurotrauma 2014, 31, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.; Beems, T.; Stulemeijer, M.; van Vugt, A.B.; van der Vliet, T.M.; Borm, G.F.; Vos, P.E. Outcome prediction in mild traumatic brain injury: Age and clinical variables are stronger predictors than CT abnormalities. J. Neurotrauma 2010, 27, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Botes, R.; Vermeulen, K.M.; Gerber, A.M.; Ranchor, A.V.; Buskens, E. Health-related quality of life and well-being health state values among Dutch oldest old. Patient Prefer. Adherence 2019, 13, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Liu, D.; Wei, L.; Wen, Y.; Lin, H.; Chen, H.; Chiu, H. Postconcussion Symptoms After an Uncomplicated Mild Traumatic Brain Injury in Older Adults: Frequency, Risk Factors, and Impact on Quality of Life. J. Head Trauma Rehabil. 2022, 37, 278–284. [Google Scholar] [CrossRef]
- Available online: https://www.rivm.nl/monitor-langer-thuis/resultaten/percentage-75-plussers-met-goede-kwaliteit-van-leven (accessed on 1 April 2024).


| Total (n = 453) | Older Patients (n = 164) | Younger Patients (n = 289) | p-Value * | Missing | |
|---|---|---|---|---|---|
| Age, mean ± sd | 49.2 ± 18.2 | 68.6 ± 7.0 | 39.2 ± 13.4 | <0.001 | 0 |
| Sex (male), n (%) | 270 (59.6) | 98 (59.8) | 172 (59.5) | 0.96 | 0 |
| Comorbidities, n (%) | 155 (34.2) | 105 (64.0) | 50 (17.3) | <0.001 | 0 |
| GCS = 15, n (%) | 297 (65.6) | 116 (70.7) | 181 (62.6) | 0.07 | 0 |
| Hospital admission, n (%) | 283 (62.5) | 113 (68.9) | 170 (58.8) | 0.03 | 0 |
| Mechanism of injury, n (%) | 0.35 | 0 | |||
| Collision | 108 (23.8) | 31 (18.9) | 77 (26.6) | ||
| Fall | 309 (68.2) | 124 (75.6) | 185 (64.0) | ||
| Other cause | 36 (7.9) | 9 (5.5) | 27 (9.3) | ||
| Discharged to home, n (%) | 434 (95.8) | 157 (95.7) | 277 (95.8) | 0.95 | 0 |
| ISS, mean ± sd | 7.3 ± 5.2 | 7.7 ± 5.2 | 7.2 ± 5.2 | 0.38 | 46 |
| CT abnormalities, n (%) | 78 (17.2) | 33 (20.1) | 45 (15.6) | 0.22 | 0 |
| Pre-injury physical †, n (%) | 112 (24.7) | 56 (34.1) | 56 (19.4) | <0.001 | 15 |
| Pre-injury mental health †, n (%) | 33 (7.3) | 11 (6.7) | 22 (7.6) | 0.80 | 17 |
| Educational level high, n (%) | 220 (50.5) | 64 (42.1) | 156 (54.9) | 0.01 | 17 |
| Living alone, n (%) | 86 (19.5) | 29 (18.5) | 57 (20.1) | 0.60 | 12 |
| Answer Scale | Perception of HRQoL | Satisfaction with Health | ||||
|---|---|---|---|---|---|---|
| Older Patients (n = 164) | Younger Patients (n = 288 *) | p-Value | Older Patients (n = 164) | Younger Patients (n = 288 *) | p-Value | |
| Poor (1–3) | 33 (20.1) | 62 (21.5) | 0.724 | 72 (43.9) | 97 (33.7) | 0.031 |
| Good (4–5) | 131 (79.9) | 226 (78.5) | 92 (56.1) | 191 (66.3) | ||
| Health-Related Quality of Life | ||
|---|---|---|
| Poor | Good | |
| Total group (N = 439) | ||
| Incomplete recovery | 74 (41.8%) | 103 (58.2%) |
| Complete recovery | 19 (7.3%) | 243 (92.7%) |
| Older patients (N = 161) | ||
| Incomplete recovery | 23 (33.3%) | 46 (66.7%) |
| Complete recovery | 10 (10.9%) | 82 (89.1%) |
| Younger patients (N = 278) | ||
| Incomplete recovery | 51 (47.2%) | 57 (52.8%) |
| Complete recovery | 9 (5.3%) | 161 (94.7%) |
| Poor One-Year Post-Injury HRQoL Perception of Older Patients (N = 164) | |||||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | ||||||
| Coding | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Baseline Data | |||||||
| Sex | Male (0)–Female (1) | 0.82 | 0.37–1.80 | 0.61 | NS | NS | NS |
| Education level | Low (0)–High (1) | 0.67 | 0.30–1.49 | 0.33 | NS | NS | NS |
| Living alone | No (0)–Yes (1) | 2.56 | 1.06–6.23 | 0.04 * | NS | NS | NS |
| Pre-injury physical complaints | No (0)–Yes (1) | 1.77 | 0.81–3.84 | 0.15 | NS | NS | NS |
| Pre-injury mental health | No (0)–Yes (1) | 0.88 | 0.18–4.26 | 0.87 | NS | NS | NS |
| GCS score ED | <15 (0)–15 (1) | 1.06 | 0.46–2.45 | 0.88 | NS | NS | NS |
| CT abnormalities | No (0)–Yes (1) | 0.86 | 0.32–2.82 | 0.76 | NS | NS | NS |
| ISS score | 0–39 | 0.94 | 0.85–1.04 | 0.20 | NS | NS | NS |
| Two weeks post-injury | |||||||
| Active coping style | No (0)–Yes (1) | 0.52 | 0.18–1.46 | 0.21 | NS | NS | NS |
| Passive coping style | No (0)–Yes (1) | 1.08 | 0.40–2.93 | 0.89 | NS | NS | NS |
| Avoidant coping style | No (0)–Yes (1) | 0.59 | 0.22–1.57 | 0.29 | NS | NS | NS |
| Post-traumatic complaints | 0–25 | 1.17 | 1.07–1.29 | 0.01 * | NS | NS | NS |
| Anxiety scores | 0–15 | 1.08 | 0.97–1.19 | 0.16 | NS | NS | NS |
| Depression scores | 0–18 | 1.21 | 1.09–1.35 | <0.01 * | 1.20 | 1.01–1.34 | <0.01 * |
| Post-traumatic stress | 0–73 | 1.01 | 0.98–1.03 | 0.64 | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coffeng, S.M.; Abdulle, A.E.; van der Horn, H.J.; de Koning, M.E.; ter Maaten, J.C.; Spikman, J.M.; van der Naalt, J. Good Health-Related Quality of Life in Older Patients One Year after mTBI despite Incomplete Recovery: An Indication of the Disability Paradox? J. Clin. Med. 2024, 13, 2655. https://doi.org/10.3390/jcm13092655
Coffeng SM, Abdulle AE, van der Horn HJ, de Koning ME, ter Maaten JC, Spikman JM, van der Naalt J. Good Health-Related Quality of Life in Older Patients One Year after mTBI despite Incomplete Recovery: An Indication of the Disability Paradox? Journal of Clinical Medicine. 2024; 13(9):2655. https://doi.org/10.3390/jcm13092655
Chicago/Turabian StyleCoffeng, Sophie M., Amaal Eman Abdulle, Harm J. van der Horn, Myrthe E. de Koning, Jan C. ter Maaten, Jacoba M. Spikman, and Joukje van der Naalt. 2024. "Good Health-Related Quality of Life in Older Patients One Year after mTBI despite Incomplete Recovery: An Indication of the Disability Paradox?" Journal of Clinical Medicine 13, no. 9: 2655. https://doi.org/10.3390/jcm13092655
APA StyleCoffeng, S. M., Abdulle, A. E., van der Horn, H. J., de Koning, M. E., ter Maaten, J. C., Spikman, J. M., & van der Naalt, J. (2024). Good Health-Related Quality of Life in Older Patients One Year after mTBI despite Incomplete Recovery: An Indication of the Disability Paradox? Journal of Clinical Medicine, 13(9), 2655. https://doi.org/10.3390/jcm13092655