Effector Mechanisms of Neutrophils within the Innate Immune System in Response to Mycobacterium tuberculosis Infection
Abstract
:1. Introduction
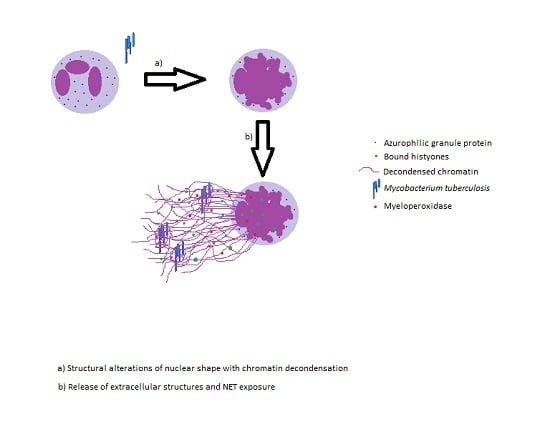
2. Neutrophil Extracellular Traps and Its Effector Functions
3. Cytokines Modulating the Functions of Neutrophils
4. Neutrophils Apoptosis and Phagocytosis Induced Cell Death
5. Genotypic Changes Affecting Neutrophil Functions
6. Neutrophil Transcriptional Changes in Chronic Diseases
7. Neutrophils and Granulomatous Responses against M. tb Infection
8. HIV Infection and Type 2 Diabetes Association with Neutrophil Immune Responses to M. tb
9. Future Directions: Developing Vaccines That Will Induce Neutrophil-Mediated Favorable Immune Responses against M. tb Infection
10. Concluding Remarks
Author Contributions
Conflicts of Interest
References
- Tuberculosis (TB). World Health Organization, 2016. Available online: http://www.who.int/tb/en/ (accessed on 4 November 2016).
- Smith, I. Mycobacterium tuberculosis Pathogenesis and Molecular Determinants of Virulence. Clin. Microbiol. Rev. 2003, 16, 463–496. [Google Scholar] [CrossRef] [PubMed]
- CDC (Centers for Disease Control and Prevention). Reported Tuberculosis in the United States, 2014; CDC Report (2014); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014.
- Natarajan, K.; Kundu, M.; Sharma, P.; Basu, J. Innate immune responses to M. tuberculosis infection. Tuberculosis 2011, 91, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Jasenosky, L.D.; Scriba, T.J.; Hanekom, W.A.; Goldfeld, A.E. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol. Rev. 2015, 264, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Yeremeev, V.; Linge, I.; Kondratieva, T.; Apt, A. Neutrophils exacerbate tuberculosis infection in genetically susceptible mice. Tuberculosis 2015, 95, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.; Nguyen, T.; Kim, J.; Kassissa, C.; Khurasany, M.; Luong, J.; Kasko, S.; Pandya, S.; Chu, M.; Chi, P.T.; et al. An elucidation of neutrophil functions against Mycobacterium tuberculosis infection. Clin. Dev. Immunol. 2013, 2013, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Hilda, J.N.; Narasimhan, M.; Das, S.D. Neutrophils from Pulmonary Tuberculosis Patients Show Augmented Levels of Chemokines MIP-1α, IL-8 and MCP-1 Which Further Increase upon in Vitro Infection with Mycobacterial Strains. Hum. Immunol. 2014, 75, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.N.; Yao, C.Y.; Jin, Q.L.; He, W.X.; Li, B.Q. The Enhanceing effect of IL-12 on phagocytosis and killing of Mycobacterium tuberculosis by neutrophils in tuberculosis patients. PMC 2011, 27, 1191–1194. [Google Scholar]
- Bainton, D.F.; Ullyot, J.L.; Farquhar, M.G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J. Exp. Med. 1971, 134, 907–934. [Google Scholar] [CrossRef] [PubMed]
- Fliedner, T.M.; Cronkite, E.P.; Robertson, J.S. Granulocytopoiesis. I. Senescence and random loss of neutrophilic granulocytes in human beings. Blood 1964, 24, 402–414. [Google Scholar] [PubMed]
- Athens, J.W.; Haab, O.P.; Raab, S.O.; Mauer, A.M.; Ashenbrucker, H.; Cartwright, G.E.; Wintrobe, M.M. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J. Clin. Investig. 1961, 40, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Zatti, M. Changes in the metabolic pattern of polymorpho-nuclear leucocytes during phagocytosis. Br. J. Exp. Pathol. 1964, 45, 548–559. [Google Scholar] [PubMed]
- Bennouna, S.; Bliss, S.K.; Curiel, T.J.; Denkers, E.Y. Cross-talk in the innate immune system: Neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J. Immunol. 2003, 171, 6052–6058. [Google Scholar] [CrossRef] [PubMed]
- Van Gisbergen, K.P.; Sanchez-Hernandez, M.; Geijtenbeek, T.B.; van Kooyk, Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J. Exp. Med. 2005, 201, 1281–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLeo, F.R. Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis 2004, 9, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.; Holzer, T.J.; Andersen, B.R. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J. Infect. Dis. 1987, 156, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.S.; Amirault, H.J.; Andersen, B.R. Killing of Mycobacterium tuberculosis by neutrophils: A nonoxidative process. J. Infect. Dis. 1990, 162, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Kisich, K.O.; Higgins, M.; Diamond, G.; Heifets, L. Tumor necrosis factor alpha stimulates killing of Mycobacterium tuberculosis by human neutrophils. Infect. Immun. 2002, 70, 4591–4599. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.J.; Butler, R.E.; Stewart, G.R. Mycobacterium tuberculosis ESAT-6 is a leukocidin causing Ca2+ influx, necrosis and neutrophil extracellular trap formation. Cell Death Dis. 2014, 5, e1474. [Google Scholar] [CrossRef] [PubMed]
- Braian, C.; Hogea, V.; Stendahl, O. Mycobacterium tuberculosis-Induced Neutrophil Extracellular Traps Activate Human Macrophages. J. Innate Immun. 2013, 5, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Zychlinsky, A. NETs: A new strategy for using old weapons. Trends Immunol. 2009, 30, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Toossi, Z. Cytokine circuits in tuberculosis. Infect. Agents Dis. 1996, 5, 98–107. [Google Scholar] [PubMed]
- Poveda, F.; Camacho, J.; Arnalich, F.; Codoceo, R.; del Arco, A.; Martinez-Hernandez, P. Circulating cytokine concentrations in tuberculosis and other chronic bacterial infections. Infection 1999, 27, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Payán, J.; Aguilar-León, D.; Lascurain-Ledezma, R.; Hernández-Pando, R. Neutrophil participation in early control and immune activation during experimental pulmonary tuberculosis. Gac. Med. Mex. 2006, 142, 273–281. [Google Scholar] [PubMed]
- Tsuda, Y.; Takahashi, H.; Kobayashi, M.; Hanafusa, T.; Herndon, D.N.; Suzuki, F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 2004, 21, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Appelberg, R.; Castro, A.G.; Pedrosa, J.; Minoprio, P. Role of interleukin-6 in the induction of protective T cells during mycobacterial infections in mice. Immunology 1994, 82, 361–364. [Google Scholar] [PubMed]
- Bermudez, L.E.; Young, L.S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-γ, is associated with macrophage killing of Mycobacterium avium complex. J. Immunol. 1988, 140, 3006–3013. [Google Scholar] [PubMed]
- Champsi, J.; Young, L.S.; Bermudez, L.E. Production of TNF-α, IL-6 and TGF-β, and expression of receptors for TNF-α and IL-6, during murine Mycobacterium avium infection. Immunology 1995, 84, 549–554. [Google Scholar] [PubMed]
- Dunlap, N.E.; Briles, D.E. Immunology of tuberculosis. Med. Clin. N. Am. 1993, 77, 1235–1251. [Google Scholar] [CrossRef]
- Falcone, V.; Bassey, E.B.; Toniolo, A.; Conaldi, P.G.; Collins, F.M. Differential release of tumor necrosis factor-α from murine peritoneal macrophages stimulated with virulent and avirulent species of mycobacteria. FEMS Immunol. Med. Microbiol. 1994, 8, 225–232. [Google Scholar] [PubMed]
- Fiorenza, G.; Bottasso, O.A.; Rateni, L.; Farroni, M.A.; Dlugovitzky, D. Impaired neutrophil function in patients with pulmonary tuberculosis and its normalization in those undergoing specific treatment, except the HIV-coinfected cases. FEMS Immunol. Med. Microbiol. 2003, 35, 159–164. [Google Scholar] [CrossRef]
- Riedel, D.D.; Kaufmann, S.H. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect. Immun. 1997, 65, 4620–4623. [Google Scholar] [PubMed]
- Schluger, N.W.; Rom, W.N. The host immune response to tuberculosis. Am. J. Respir. Crit. Care Med. 1998, 157, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Strieter, R.M.; Kasahara, K.; Allen, R.M.; Standiford, T.J.; Rolfe, M.W.; Becker, F.S.; Chensue, S.W.; Kunkel, S.L. Cytokine-induced neutrophil-derived interleukin-8. Am. J. Pathol. 1992, 141, 397–407. [Google Scholar] [PubMed]
- Bazzoni, F.; Cassatella, M.A.; Rossi, F.; Ceska, M.; Dewald, B.; Baggiolini, M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J. Exp. Med. 1991, 173, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Friedland, J.S.; Hartley, J.C.; Hartley, C.G.; Shattock, R.J.; Griffin, G.E. Cytokine secretion in vivo and ex vivo following chemotherapy of Mycobacterium tuberculosis infection. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 199–203. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, H.D.; Wang, Y.; Wang, Q.; Li, Y.; Zhao, Y.; Zhang, X.L. Interleukin 24 as a novel potential cytokine immunotherapy for the treatment of Mycobacterium tuberculosis infection. Microbes Infect. 2011, 13, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Pokkali, S.; Rajavelu, P.; Sudhakar, R.; Das, S.D. Phenotypic modulation in Mycobacterium tuberculosis infected neutrophil during tuberculosis. Indian J. Med. Res. 2009, 130, 185–192. [Google Scholar] [PubMed]
- Nandi, B.; Behar, S.M. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J. Exp. Med. 2011, 208, 2251–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velmurugan, K.; Chen, B.; Miller, J.L.; Azogue, S.; Gurses, S.; Hsu, T.; Glickman, M.; Jacobs, W.R., Jr.; Porcelli, S.A.; Briken, V. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog. 2007, 3, e110. [Google Scholar] [CrossRef] [PubMed]
- Blomgran, R.; Desvignes, L.; Briken, V.; Ernst, J.D. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe 2012, 11, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Blomgran, R.; Ernst, J.D. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J. Immunol. 2011, 186, 7110–7119. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.I.; Kim, M. A day (or 5) in a neutrophil’s life. Blood 2010, 116, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Braughton, K.R.; Palazzolo-Ballance, A.M.; Kennedy, A.D.; Sampaio, E.; Kristosturyan, E.; Whitney, A.R.; Sturdevant, D.E.; Dorward, D.W.; Holland, S.M.; et al. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J. Innate Immun. 2010, 2, 560–575. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.M.; Allen, L.A. Regulation of human neutrophil apoptosis and lifespan in health and disease. J. Cell Death 2014, 7, 15–23. [Google Scholar] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Henson, P.M. Phagocyte receptors for apoptotic cells: Recognition, uptake, and consequences. J. Clin. Investig. 2001, 108, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; DeLeo, F.R. Towards a comprehensive understanding of the role of neutrophils in innate immunity: A systems biology-level approach. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gilbert, G.E.; Kokubo, Y.; Ohashi, T. Role of the liver in regulating numbers of circulating neutrophils. Blood 2001, 98, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Braughton, K.R.; Whitney, A.R.; Voyich, J.M.; Schwan, T.G.; Musser, J.M.; DeLeo, F.R. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc. Natl. Acad. Sci. USA 2003, 100, 10948–10953. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Voyich, J.M.; Braughton, K.R.; DeLeo, F.R. Down-regulation of proinflammatory capacity during apoptosis in human polymorphonuclear leukocytes. J. Immunol. 2003, 170, 3357–3368. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Voll, R.E.; Herrmann, M.; Roth, E.A.; Stach, C.; Kalden, J.R.; Girkontaite, I. Immuno-suppressive effects of apoptotic cells. Nature 1997, 390, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Korns, D.; Frasch, S.C.; Fernandez-Boyanapalli, R.; Henson, P.M.; Bratton, D.L. Modulation of macrophage efferocytosis in inflammation. Front. Immunol. 2011, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- Eruslanov, E.B.; Lyadova, I.V.; Kondratieva, T.K.; Konstantin, K.B.; Scheglov, I.V.; Orlova, M.O.; Apt, A.S. Neutrophil Responses to Mycobacterium tuberculosis Infection in Genetically Susceptible and Resistant Mice. Infect. Immun. 2005, 73, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.P.; Graham, C.M.; McNab, F.W.; Susanna, Z.X.; O’Garra, A. An Interferon-Inducible Neutrophil-Driven Blood Transcriptional Signature in Human Tuberculosis. Nature 2010, 466, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Bozzano, F.; Marras, F.; De Maria, A. Immunology of tuberculosis. Mediterr. J. Hematol. Infect. Dis. 2014, 6, e2014027. [Google Scholar] [CrossRef] [PubMed]
- Cardona, P.J.; Prats, C. The Small Breathing Amplitude at the Upper Lobes Favors the Attraction of Polymorphonuclear Neutrophils to Mycobacterium tuberculosis Lesions and Helps to Understand the Evolution toward Active Disease in An Individual-Based Model. Front. Microbiol. 2016, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ly, J.; Lagman, M.; Saing, T.; Singh, M.K.; Tudela, E.V.; Morris, D.; Anderson, J.; Daliva, J.; Ochoa, C.; Patel, N.; et al. Liposomal GSH Supplementation Restores TH1 Cytokine Response to Mycobacterium tuberculosis Infection in HIV-Infected Individuals. J. Interferon Cytokine Res. 2015, 35, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Conradie, F.; Foulkes, A.S.; Ive, P.; Yin, X.; Roussos, K.; Glencross, D.K.; Lawrie, D.; Stevens, W.; Montaner, L.J.; Sanne, I.; et al. Natural killer cell activation distinguishes Mycobacterium tuberculosis-mediated immune reconstitution syndrome from chronic HIV and HIV/MTB coinfection. J. Acquir. Immune Defic. Syndr. 2011, 58, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Trapani, J.A.; Smyth, M.J. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2002, 2, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.M.; Redford, P.S.; Wilkinson, R.J.; O’Garra, A.; Martineau, A.R. Neutrophils in tuberculosis: Friend or foe? Trends Immunol. 2012, 33, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Bangani, N.; Nakiwala, J.; Martineau, A.R.; Wilkinson, R.J.; Wilkinson, K.A.; Lowe, D.M. Brief report: HIV-1 infection impairs CD16 and CD35 mediated opsonophagocytosis of Mycobacterium tuberculosis by human neutrophils. J. Acquir. Immune Defic. Syndr. 2016, 73, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Bailey, C.; Cahatol, I.; Dodge, L.; Yim, J.; Kassissa, C.; Luong, J.; Kasko, S.; Pandya, S.; Venketaraman, V. Mechanisms of control of Mycobacterium tuberculosis by NK cells: Role of glutathione. Front. Immunol. 2015, 6, 508. [Google Scholar] [CrossRef] [PubMed]
- Whalen, C.; Horsburgh, C.R.; Hom, D.; Lahart, C.; Simberkoff, M.; Ellner, J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am. J. Respir. Crit. Care Med. 1995, 151, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.L.; Goldstein, M.M.; Chan, J.; Triebold, K.J.; Pfeffer, K.; Lowenstein, C.J.; Schreiber, R.; Mak, T.W.; Bloom, B.R. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995, 2, 561–572. [Google Scholar] [CrossRef]
- Lachmandad, E.; Heuvel, C.; Damen, M.; Cleophas, M.; Netea, M.G.; van Crevel, R. Diabetes mellitus and increased tuberculosis susceptibility: The role of short-chain fatty acids. J. Diabetes Res. 2016, 2016, 6014631. [Google Scholar]
- Vinolo, M.A.; Hatanaka, E.; Lambertucci, R.H.; Newsholme, P.; Curi, R. Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell Biochem. Funct. 2009, 27, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Agarkov, A.A.; Popova, T.N.; Verevkin, A.N.; Matasova, L.V. Activity of the glutathione antioxidant system and NADPH-generating enzymes in blood serum of rats with Type 2 diabetes mellitus after administration of melatonin-correcting drugs. Bull. Exp. Biol. Med. 2014, 157, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.M.; Folgado, J.; Tormo, C.; Artero, A.; Ascaso, M.; Martinez-Hervás, S.; Chaves, F.J.; Ascaso, J.F.; Real, J.T. Altered glutathione system is associated with the presence of distal symmetric peripheral polyneuropathy in Type 2 diabetic subjects. J. Diabetes Complicat. 2015, 29, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.M.; Tretini, M.M.; Junquiera-Kipnis, A.P.; Kipnis, A. The mc2-CMX vaccine induced an enhanced immune response against Mycobacterium tuberculosis compared to Bacillus Calmette-Guérin but with similar lung inflammatory effects. Mem. Inst. Oswaldo Cruz. 2016, 111, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Trentini, M.M.; de Oliveira, F.M.; Kipnis, A.; Junqueira-Kipnis, A.P. The role of neutrophils in the induction of specific Th1 and Th17 during Vaccination against tuberculosis. Front. Microbiol. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warren, E.; Teskey, G.; Venketaraman, V. Effector Mechanisms of Neutrophils within the Innate Immune System in Response to Mycobacterium tuberculosis Infection. J. Clin. Med. 2017, 6, 15. https://doi.org/10.3390/jcm6020015
Warren E, Teskey G, Venketaraman V. Effector Mechanisms of Neutrophils within the Innate Immune System in Response to Mycobacterium tuberculosis Infection. Journal of Clinical Medicine. 2017; 6(2):15. https://doi.org/10.3390/jcm6020015
Chicago/Turabian StyleWarren, Eric, Garrett Teskey, and Vishwanath Venketaraman. 2017. "Effector Mechanisms of Neutrophils within the Innate Immune System in Response to Mycobacterium tuberculosis Infection" Journal of Clinical Medicine 6, no. 2: 15. https://doi.org/10.3390/jcm6020015
APA StyleWarren, E., Teskey, G., & Venketaraman, V. (2017). Effector Mechanisms of Neutrophils within the Innate Immune System in Response to Mycobacterium tuberculosis Infection. Journal of Clinical Medicine, 6(2), 15. https://doi.org/10.3390/jcm6020015