Competing-Risk Analysis of Death and End Stage Kidney Disease by Hyperkalaemia Status in Non-Dialysis Chronic Kidney Disease Patients Receiving Stable Nephrology Care
Abstract
:1. Introduction
2. Materials and Methods
Statistics
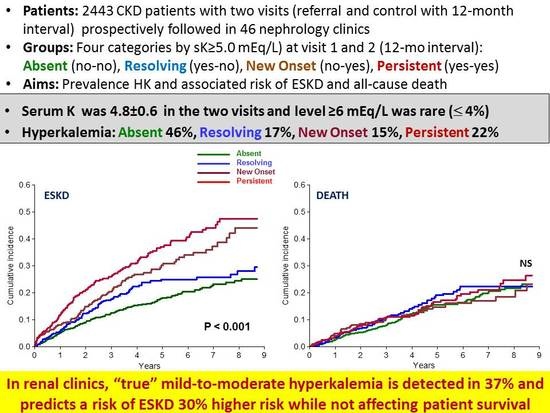
3. Results
3.1. Baseline Period
3.2. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hayes, J.; Kalantar-Zadeh, K.; Lu, J.L.; Turban, S.; Anderson, J.E.; Kovesdy, C.P. Association of hypo- and hyperkalaemia with disease progression and mortality in males with chronic kidney diseasehe role of race. Nephron Clin. Pract. 2012, 120, c8–c16. [Google Scholar] [CrossRef] [PubMed]
- Drawz, P.E.; Babineau, D.C.; Rahman, M. Metabolic complications in elderly adults with chronic kidney disease. J. Am. Geriatr. Soc. 2012, 60, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Blacklock, R.; Wood, E.; Rumjon, A.; Simmonds, S.; Fletcher-Rogers, J.; Ariyanayagam, R.; Al-Yassin, A.; Sharpe, C.; Vinen, K. Prevalence and factors associated with hyperkalaemia in predialysis patients followed in a low-clearance clinic. Clin. J. Am. Soc. Nephrol. 2012, 7, 1234–1241. [Google Scholar] [CrossRef]
- Nakhoul, G.N.; Huang, H.; Arrigain, S.; Jolly, S.E.; Schold, J.D.; Nally, Jr.J.V.; Navaneethan, S.D. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am. J. Nephrol. 2015, 41, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Brunelli, S.M.; Jensen, D.E.; Yang, A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin. J. Am. Soc. Nephrol. 2016, 11, 90–100. [Google Scholar] [CrossRef]
- Chang, A.R.; Sang, Y.; Leddy, J.; Yahya, T.; Kirchner, H.L.; Inker, L.A.; Matsushita, K.; Ballew, S.H.; Coresh, J.; Grams, M.E. Antihypertensive medications and the prevalence of hyperkalaemia in a large health system. Hypertension 2016, 67, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Pitt, B.; Reaven, N.; Funk, S.; McGaughey, K.; Wilson, D.; Bushinsky, D.A. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am. J. Nephrol. 2017, 46, 213–221. [Google Scholar] [CrossRef]
- Hughes-Austin, J.M.; Rifkin, D.E.; Beben, T.; Katz, R.; Sarnak, M.J.; Deo, R.; Hoofnagle, A.N.; Homma, S.; Siscovick, D.S.; Sotoodehnia, N.; et al. The relation of serum potassium concentration with cardiovascular events and mortality in community-living individuals. Clin. J. Am. Soc. Nephrol. 2017, 12, 245–252. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, A.R.; McAdams DeMarco, M.A.; Inker, L.A.; Matsushita, K.; Ballew, S.H.; Coresh, J.; Grams, M.E. Serum potassium, mortality and kidney outcomes in the atherosclerosis risk in communities study. Mayo Clin. Proc. 2016, 91, 1403–1412. [Google Scholar] [CrossRef]
- Korgaonkar, S.; Tilea, A.; Gillespie, B.W.; Kiser, M.; Eisele, G.; Finkelstein, F.; Kotanko, P.; Pitt, B.; Saran, R. Serum potassium and outcomes in CKD: Insights from the RRI-CKD cohort study. Clin. J. Am. Soc. Nephrol. 2010, 5, 762–769. [Google Scholar] [CrossRef]
- Chen, Y.; Sang, Y.; Ballew, S.H.; Tin, A.; Chang, A.R.; Matsushita, K.; Coresh, J.; Kalantar-Zadeh, K.; Molnar, M.Z.; Grams, M.E. Race, Serum potassium and associations with ESRD and mortality. Am. J. Kidney Dis. 2017, 70, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.W.; Nicolaisen, S.K.; Hasvold, P.; Garcia-Sanchez, R.; Pedersen, L.; Adelborg, K.; Egfjord, M.; Egstrup, K.; Sørensen, H.T. Elevated potassium levels in patients with chronic kidney diseaseccurrence, risk factors and clinical outcomes-a Danish population-based cohort study. Nephrol. Dial. Transplant. 2017. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Matsushita, K.; Sang, Y.; Brunskill, N.J.; Carrero, J.J.; Chodick, G.; Hasegawa, T.; Heerspink, H.L.; Hirayama, A.; Landman, G.W.D.; et al.; CKD Prognosis Consortium Serum potassium and adverse outcomes across the range of kidney function: A CKD Prognosis Consortium meta-analysis. Eur. Heart J. 2018, 39, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Hung, C.C.; Hwang, D.Y.; Kuo, M.C.; Chiu, Y.W.; Chang, J.M.; Tsai, J.C.; Hwang, S.J.; Seifter, J.L.; Chen, H.C. Hypokalemia, its contributing factors and renal outcomes in patients with chronic kidney disease. PLoS ONE 2013, 8, e67140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, S.; Metzger, M.; Flamant, M.; Houillier, P.; Haymann, J.P.; Vrtovsnik, F.; Thervet, E.; Boffa, J.J.; Massy, Z.A.; Stengel, B.; et al.; NephroTest Study group Association of plasma potassium with mortality and end-stage kidney disease in patients with chronic kidney disease under nephrologist care—The NephroTest study. BMC Nephrol. 2017, 18, 295. [Google Scholar]
- Bandak, G.; Sang, Y.; Gasparini, A.; Chang, A.R.; Ballew, S.H.; Evans, M.; Arnlov, J.; Lund, L.H.; Inker, L.A.; Coresh, J.; et al. Hyperkalaemia after initiating renin-angiotensin system blockade: The Stockholm Creatinine Measurements (SCREAM) project. J. Am. Heart Assoc. 2017, 6, e005428. [Google Scholar] [CrossRef] [PubMed]
- Georgianos, P.I.; Agarwal, R. Revisiting RAAS blockade in CKD with newer potassium-binding drugs. Kidney Int. 2018, 93, 325–334. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Y.; Perkovic, V.; Li, X.; Ninomiya, T.; Hou, W.; Zhao, N.; Liu, L.; Lv, J.; Zhang, H.; et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: A bayesian network meta-analysis of randomized clinical trials. Am. J. Kidney. Dis. 2016, 67, 728–741. [Google Scholar] [CrossRef]
- Epstein, M.; Reaven, N.L.; Funk, S.E.; McGaughey, K.J.; Oestreicher, N.; Knispel, J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am. J. Manag. Care 2015, 21, S212–220. [Google Scholar]
- Cupisti, A.; Kovesdy, C.P.; D’Alessandro, C.; Kalantar-Zadeh, K. Dietary approach to recurrent or chronic hyperkalaemia in patients with decreased kidney function. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Roscioni, S.S.; Lambers Heerspink, H.J. Clinical trials: New nonabsorbable potassium-exchange resins in hyperkalaemia. Nat. Rev. Nephrol. 2015, 11, 205–206. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, L.; Di Lullo, L.; Paoletti, E.; Cupisti, A.; Bianchi, S. Chronic hyperkalaemia in non-dialysis CKD: Controversial issues in nephrology practice. J. Nephrol. 2018. [Google Scholar] [CrossRef]
- De Nicola, L.; Chiodini, P.; Zoccali, C.; Borrelli, S.; Cianciaruso, B.; Di Iorio, B.; Santoro, D.; Giancaspro, V.; Abaterusso, C.; Gallo, C.; et al.; SIN-TABLE CKD study group Prognosis of CKD patients receiving outpatient nephrology care in Italy. Clin. J. Am. Soc. Nephrol. 2011, 6, 2421–2428. [Google Scholar] [CrossRef]
- Brück, K.; Jager, K.J.; Zoccali, C.; Bello, A.K.; Minutolo, R.; Ioannou, K.; Verbeke, F.; Völzke, H.; Arnlöv, J.; Leonardis, D.; European CKD Burden Consortium. Different rates of progression and mortality in patients with chronic kidney disease at outpatient nephrology clinics across Europe. Kidney Int. 2018, 93, 1432–1441. [Google Scholar]
- De Nicola, L.; Provenzano, M.; Chiodini, P.; Borrelli, S.; Garofalo, C.; Pacilio, M.; Liberti, ME.; Sagliocca, A.; Conte, G.; Minutolo, R. Independent role of underlying kidney disease on renal prognosis of patients with chronic kidney disease under nephrology care. PLoS ONE 2015, 10, e0127071. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, R.; Locatelli, F.; Gallieni, M.; Bonofiglio, R.; Fuiano, G.; Oldrizzi, L.; Conte, G.; De Nicola, L.; Mangione, F.; Esposito, P.; et al.; REport of COmorbidities in non-Dialysis Renal Disease Population in Italy (RECORD-IT) Study Group Anaemia management in non-dialysis Chronic Kidney Disease (CKD) patients multicentre prospective study in renal clinics. Nephrol. Dial. Transplant. 2013, 28, 3035–3045. [Google Scholar] [CrossRef] [PubMed]
- Cianciaruso, B.; Pota, A.; Bellizzi, V.; Di Giuseppe, D.; Di Micco, L.; Minutolo, R.; Pisani, A.; Sabbatini, M.; Ravani, P. Effect of a low-versus moderate-protein diet on progression of CKD: Follow-up of a randomized controlled trial. Am. J. Kidney Dis. 2009, 54, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Kochar, S.C.; Lam, K.F.; Yip, P.S.F. Generalized supremum tests for the equality of cause specific hazard rates. Lifetime Data Anal. 2002, 8, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Lee, D.S.; Fine, J.P. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016, 133, 601–609. [Google Scholar] [CrossRef]
- Gray, R.J. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 1988, 16, 1141–1154. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Jones, C.; Roderick, P.; Harris, S.; Rogerson, M. Decline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney disease. Nephrol. Dial. Transplant. 2006, 21, 2133–2143. [Google Scholar] [CrossRef] [Green Version]
- Ruggenenti, P.; Perticucci, E.; Cravedi, P.; Gambara, V.; Costantini, M.; Sharma, S.K.; Perna, A.; Remuzzi, G. Role of remission clinics in the longitudinal treatment of CKD. J. Am. Soc. Nephrol. 2008, 19, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.L.; Kern, E.F.; Miller, D.R.; Tiwari, A.; Maney, M.; Rajan, M.; Pogach, L. Survival benefit of nephrologic care in patients with diabetes mellitus and chronic kidney disease. Survival benefit of nephrologic care in patients with diabetes mellitus and chronic kidney disease. Arch. Intern. Med. 2008, 168, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.A.; Titus, T.T. Outcomes of early versus late nephrology referral in chronic kidney disease: A systematic review. Am. J. Med. 2011, 124, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, R.; Lapi, F.; Chiodini, P.; Simonetti, M.; Bianchini, E.; Pecchioli, S.; Cricelli, I.; Cricelli, C.; Piccinocchi, G.; Conte, G.; et al. Risk of ESRD and death in patients with CKD not referred to a nephrologist: A 7-year prospective study. Clin. J. Am. Soc. Nephrol. 2014, 9, 1586–1593. [Google Scholar] [CrossRef]
- Coresh, J. Update on the Burden of CKD. J. Am. Soc. Nephrol. 2017, 28, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Bunaye, Z.; Bekele, D.M.; Light, R.P. Competing risk factor analysis of end-stage renal disease and mortality in chronic kidney disease. Am. J. Nephrol. 2008, 28, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.; Webster, A.; Ramsay, G.; Morgan, N.; Neary, J.; Whitworth, C.; Harty, J. Predicting mortality and uptake of renal replacement therapy in patients with stage 4 chronic kidney disease. Nephrol. Dial. Transplant. 2009, 24, 1930–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obi, Y.; Kimura, T.; Nagasawa, Y.; Yamamoto, R.; Yasuda, K.; Sasaki, K.; Kitamura, H.; Imai, E.; Rakugi, H.; Isaka, Y.; et al. Impact of age and overt proteinuria on outcomes of stage 3 to 5 chronic kidney disease in a referred cohort. Clin. J. Am. Soc. Nephrol. 2010, 59, 1558–1565. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Tattersall, J.; Dekker, F.; Heimbürger, O.; Jager, K.J.; Lameire, N.; Lindley, E.; Van Biesen, W.; Vanholder, R.; Zoccali, C. ERBP Advisory Board. When to start dialysispdated guidance following publication of the Initiating Dialysis Early and Late (IDEAL) study. Nephrol. Dial. Transplant. 2011, 26, 2082–2086. [Google Scholar] [CrossRef]
- van de Luijtgaarden, M.W.; Noordzij, M.; Tomson, C.; Couchoud, C.; Cancarini, G.; Ansell, D.; Bos, W.J.; Dekker, F.W.; Gorriz, J.L.; Iatrou, C.; et al. Factors influencing the decision to start renal replacement therapy: Results of a survey among European nephrologists. Am. J. Kidney Dis. 2012, 60, 940–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, S.C.; Mavridis, D.; Navarese, E.; Craig, J.C.; Tonelli, M.; Salanti, G.; Wiebe, N.; Ruospo, M.; Wheeler, D.C.; Strippoli, G.F. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease network meta-analysis. Lancet 2015, 385, 2047–2056. [Google Scholar] [CrossRef]
- Voskamp, P.W.M.; Dekker, F.W.; van Diepen, M.; Hoogeveen, E.K.; PREPARE-2 Study Group. Effect of dual compared to no or single renin-angiotensin system blockade on risk of renal replacement therapy or death in predialysis patients: PREPARE-2 study. J. Am. Soc. Hypertens. 2017, 11, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R.; Lakkis, J.I.; Jaar, B.; Rocco, M.V.; Choi, M.J.; Kramer, H.J.; Ku, E. Use of Renin-Angiotensin System Blockade in Advanced CKD: An NKF-KDOQI Controversies Report. Am. J. Kidney Dis. 2018, 72, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Dobre, D.; Heerspink, H.J.; Brenner, B.M.; Cooper, M.E.; Parving, H.H.; Shahinfar, S.; Grobbee, D.; de Zeeuw, D. Increased serum potassium affects renal outcomes: A post hoc analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. Diabetologia 2011, 54, 44–50. [Google Scholar] [CrossRef]
- Weir, M.R.; Bushinsky, D.A.; Benton, W.W.; Woods, S.D.; Mayo, M.R.; Arthur, S.P.; Pitt, B.; Bakris, G.L. Effect of patiromer on hyperkalaemia recurrence in older chronic kidney disease patients taking RAAS inhibitors. Am. J. Med. 2018, 131, 555–564. [Google Scholar] [CrossRef]




| Hyperkalaemia | p | |||||
|---|---|---|---|---|---|---|
| Overall | Absent (No-No) | Resolving (Yes-No) | New Onset (No-Yes) | Persistent (Yes-Yes) | ||
| Number (%) | 2443 (100) | 1121 (46) | 415 (17) | 363 (15) | 544 (22) | |
| Age (years) | 65.1 ± 14.6 | 64.4 ± 15.2 | 66.6 ± 13.6 | 63.6 ± 15.4 | 66.4 ± 13.4 | 0.01 |
| Males (%) | 58 | 57 | 58 | 64 | 59 | 0.12 |
| BMI (kg/m2) | 27.8 ± 5.1 | 27.7 ± 5.0 | 27.8 ± 5.1 | 27.5 ± 4.8 | 28.1 ± 5.4 | 0.35 |
| Diabetes (%) | 28 | 26 | 28 | 29 | 31 | 0.15 |
| CV disease (%) | 36 | 35 | 37 | 36 | 36 | 0.86 |
| Smoking (%) | 13 | 13 | 15 | 13 | 12 | 0.68 |
| eGFR (mL/min/1.73 m2) | 35.0 ± 17.3 | 39.0 ± 19.3 | 33.1 ± 15.0 | 34.3 ± 16.7 | 28.6 ± 12.3 | <0.001 |
| Systolic BP (mmHg) | 139 ± 20 | 139 ± 20 | 139 ± 20 | 139 ± 21 | 141 ± 19 | 0.281 |
| Renal disease (%) | <0.001 | |||||
| HTN | 30 | 33 | 32 | 28 | 24 | |
| DKD | 13 | 10 | 13 | 16 | 17 | |
| GN | 17 | 19 | 15 | 15 | 15 | |
| TIN | 9 | 10 | 9 | 10 | 8 | |
| PKD | 5 | 5 | 5 | 5 | 6 | |
| Other | 7 | 7 | 6 | 8 | 9 | |
| Unknow | 19 | 17 | 20 | 19 | 22 | |
| Absent (No-No) (n = 1121) | Resolving (Yes-No) (n = 415) | New Onset (No-Yes) (n = 363) | Persistent (Yes-Yes) (n = 544) | |||||
|---|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 1 | Visit 2 | Visit 1 | Visit 2 | Visit 1 | Visit 2 | |
| Systolic BP (mmHg) | 139 ± 20 | 135 ± 19 * | 139 ± 20 | 136 ± 19 * | 139 ± 21 | 134 ± 19 * | 141 ± 19 | 137 ± 18 * |
| Diastolic BP (mmHg) | 80 ± 11 | 78 ± 11 * | 79 ± 11 | 77 ± 10 * | 80 ± 11 | 77 ± 10 * | 79 ± 11 | 77 ± 10 * |
| Potassium (mEq/L) | 4.38 ± 0.40 | 4.40 ± 0.37 | 5.32 ± 0.35 | 4.55 ± 0.31 | 4.57 ± 0.32 | 5.30 ± 0.30 | 5.47 ± 0.44 | 5.46 ± 0.42 |
| Glucose (mg/dL) | 104.4 ± 33.4 | 103.9 ± 32.3 | 112.6 ± 48.6 | 110.9 ± 39.6 | 106.7 ± 36.9 | 108.6 ± 43.5 | 108.9 ± 42.9 | 107.7 ± 37.4 |
| Phosphate (mg/dL) ^ | 3.67 ± 0.78 | 3.71 ± 1.11 | 3.84 ± 0.75 | 3.78 ± 0.77 | 3.77 ± 0.74 | 3.84 ± 0.95 | 3.95 ± 0.79 | 4.02 ± 0.88 * |
| Haemoglobin (g/dL) ^ | 13.0 ± 1.8 | 12.9 ± 1.7 * | 12.7 ± 1.8 | 12.7 ± 1.7 | 12.5 ± 1.7 | 12.5 ± 1.7 | 12.2 ± 1.7 | 12.2 ± 1.6 |
| eGFR (mL/min/1.73 m2) ^ | 39.0 ± 19.3 | 38.3 ± 20.3 * | 33.1 ± 15.0 | 33.6 ± 17.1 | 34.3 ± 16.7 | 31.4 ± 16.6 * | 28.6 ± 12.3 | 25.8 ± 12.3 * |
| eGFR change (mL/min/year) ^ | −0.55 ± 10.66 | 1.13 ± 10.34 | −2.93 ± 9.63 | −2.90 ± 7.81 | ||||
| Proteinuria (g/24 h) ^ | 0.30 (0.12–1.00) | 0.28 (0.11–0.90) * | 0.36 (0.12–1.10) | 0.40 (0.12–1.06) | 0.53 (0.15–1.60) | 0.52 (0.14–1.49) | 0.60 (0.18–1.50) | 0.68 (0.19–1.45) |
| RASI ° | ||||||||
| None (%) | 33 | 34 | 28 | 33 | 33 | 31 | 23 | 30 |
| CEI or ARB (%) | 60 | 55 | 65 | 60 | 58 | 57 | 70 | 60 |
| Dual blockade (%) | 7 | 11 | 7 | 7 | 10 | 13 | 7 | 10 |
| Hyperkalaemia | ESKD | All-Cause Death | ||||
|---|---|---|---|---|---|---|
| Incidence (Events/Pts) | Incidence Rate per 100-pt-y (95%-CI) | sHR (95%-CI) | Incidence (Events/Patients) | Incidence Rate per 100-pt-y (95%-CI) | sHR (95%-CI) | |
| Absent | 188/1121 | 4.32 (3.72–4.98) | Reference | 147/1121 | 3.38 (2.85–3.97) | Reference |
| Resolving | 93/415 | 5.98 (4.83–7.33) | 0.98 (0.72–1.33) | 65/415 | 4.18 (3.23–5.33) | 1.01 (0.73–1.36) |
| New-onset | 105/363 | 8.23 (6.73–9.96) | 1.34 (1.05–1.72) | 51/363 | 4.00 (2.98–5.26) | 0.94 (0.64–1.38) |
| Persistent | 181/544 | 10.52 (9.04–12.17) | 1.27 (1.02–1.58) | 86/544 | 5.00 (3.99–6.17) | 0.91 (0.67–1.25) |
| Hyperkalaemia | RASI | Incidence (events/pts) | Incidence Rate (per 100-pts-y) | sHR (95% CI) | p |
|---|---|---|---|---|---|
| Absent/Resolving | Yes | 137/1.018 | 3.18 (2.67–3.75) | Reference | - |
| Absent/Resolving | No | 144/518 | 9.02 (7.61–10.62) | 1.09 (0.83–1.44) | 0.540 |
| New onset/Persistent | Yes | 171/632 | 7.43 (6.36–8.63) | 1.18 (0.94–1.49) | 0.140 |
| New onset/Persistent | No | 115/275 | 16.55 (13.67–19.87) | 1.57 (1.20–2.06) | 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Provenzano, M.; Minutolo, R.; Chiodini, P.; Bellizzi, V.; Nappi, F.; Russo, D.; Borrelli, S.; Garofalo, C.; Iodice, C.; De Stefano, T.; et al. Competing-Risk Analysis of Death and End Stage Kidney Disease by Hyperkalaemia Status in Non-Dialysis Chronic Kidney Disease Patients Receiving Stable Nephrology Care. J. Clin. Med. 2018, 7, 499. https://doi.org/10.3390/jcm7120499
Provenzano M, Minutolo R, Chiodini P, Bellizzi V, Nappi F, Russo D, Borrelli S, Garofalo C, Iodice C, De Stefano T, et al. Competing-Risk Analysis of Death and End Stage Kidney Disease by Hyperkalaemia Status in Non-Dialysis Chronic Kidney Disease Patients Receiving Stable Nephrology Care. Journal of Clinical Medicine. 2018; 7(12):499. https://doi.org/10.3390/jcm7120499
Chicago/Turabian StyleProvenzano, Michele, Roberto Minutolo, Paolo Chiodini, Vincenzo Bellizzi, Felice Nappi, Domenico Russo, Silvio Borrelli, Carlo Garofalo, Carmela Iodice, Toni De Stefano, and et al. 2018. "Competing-Risk Analysis of Death and End Stage Kidney Disease by Hyperkalaemia Status in Non-Dialysis Chronic Kidney Disease Patients Receiving Stable Nephrology Care" Journal of Clinical Medicine 7, no. 12: 499. https://doi.org/10.3390/jcm7120499
APA StyleProvenzano, M., Minutolo, R., Chiodini, P., Bellizzi, V., Nappi, F., Russo, D., Borrelli, S., Garofalo, C., Iodice, C., De Stefano, T., Conte, G., Heerspink, H. J. L., & De Nicola, L. (2018). Competing-Risk Analysis of Death and End Stage Kidney Disease by Hyperkalaemia Status in Non-Dialysis Chronic Kidney Disease Patients Receiving Stable Nephrology Care. Journal of Clinical Medicine, 7(12), 499. https://doi.org/10.3390/jcm7120499