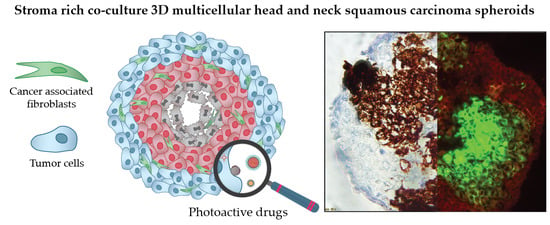
Stroma-Rich Co-Culture Multicellular Tumor Spheroids as a Tool for Photoactive Drugs Screening
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemical and Reagents
2.2. Cell Culture
2.3. Spheroids Formation
2.4. Fluorescence Staining
2.5. Analytical Techniques
2.5.1. Fluorescence Microscopy
2.5.2. Histology and Immunochemistry Analysis
2.5.3. Flow Cytometry
2.5.4. Photoirradiation
2.6. Statistics
3. Results
3.1. Formation and Characterization of Multicellular Tumor Spheroids
3.1.1. Growth Kinetics
3.1.2. Expression of Stroma Markers
3.2. Screening of Photoactive Drugs
3.2.1. Fluorescence Distribution Patterns
3.2.2. Distribution of Drugs in Homo- and Heterospheroids
3.2.3. PDT Efficacy
4. Discussion
- mTHPC. mTHPC is a highly hydrophobic and lipophilic molecule, characterized by limited penetration in tumor spheroids [57,58,59,60]. The addition of stroma had a positive outcome on the distribution of mTHPC, increasing mTHPC overall incorporation in MCTSs with a preferential accumulation in FaDu tumor cells. mTHPC releases very slowly from the cells, thus its rapid propagation across the spheroid is unlikely. This molecule usually distributes by means of serum lipoproteins in interstitial space, therefore, the enhanced penetration in heterospheroids could be related to the expansion of interstitial space in stroma-rich spheroids by stromal components. An interesting observation is that mTHPC exhibits selectivity towards FaDu tumor cells vs. CAFs. Finally, a significantly better PDT response in stroma-rich FaDu:MeWo 5:5 spheroids was obtained compared with FaDu homospheroids.
- Ce6. Contrary to mTHPC, Ce6 is more hydrophilic and penetrates more easily into spheroids [61,62,63]. Ce6 penetration in homospheroids requires 24 h, while in heterospheroids this process is accelerated and complete distribution is achieved at already 6 h. Similar to mTHPC, Ce6 possesses selectivity against FaDu tumor cells, however the overall effect of the presence of stromal components on Ce6 accumulation is negative (Ce6 accumulation in heterospheroids is lower than that in homospheroids). Thus, stroma components seem to be an additional barrier for Ce6 penetration in tumor tissue [64]. As a result, PDT efficiency of Ce6 in stroma-rich heterospheroids is not significantly different from that in homospheroids.
- ICG. An anionic, amphiphilic & water-soluble fluorophore ICG quickly distributes across any type of spheroids independently on the presence of stroma components. In contrast to PSs such as mTHPC and Ce6, ICG has no selectivity between CAFs and tumor cells.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Benien, P.; Swami, A. 3D tumor models: History, advances and future perspectives. Future Oncol. 2014, 10, 1311–1327. [Google Scholar] [CrossRef] [PubMed]
- Millard, M.; Yakavets, I.; Zorin, V.; Kulmukhamedova, A.; Marchal, S.; Bezdetnaya, L. Drug delivery to solid tumors: The predictive value of the multicellular tumor spheroid model for nanomedicine screening. Int. J. Nanomed. 2017, 12, 7993–8007. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.L. Three-dimensional in vitro cancer spheroid models for photodynamic therapy: Strengths and opportunities. Front. Phys. 2015, 3, 15. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingargiola, M.; Runge, R.; Heldt, J.-M.; Freudenberg, R.; Steinbach, J.; Cordes, N.; Baumann, M.; Kotzerke, J.; Brockhoff, G.; Kunz-Schughart, L.A. Potential of a Cetuximab-based radioimmunotherapy combined with external irradiation manifests in a 3-D cell assay. Int. J. Cancer 2014, 135, 968–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasanen, K.; Sriswasdi, S.; Valiga, A.; Tang, H.-Y.; Zhang, G.; Perego, M.; Somasundaram, R.; Li, L.; Speicher, K.; Klein-Szanto, A.J.; et al. Comparative Secretome Analysis of Epithelial and Mesenchymal Subpopulations of Head and Neck Squamous Cell Carcinoma Identifies S100A4 as a Potential Therapeutic Target. Mol. Cell. Proteom. 2013, 12, 3778–3792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.; Yang, X.; Wang, L.; Clark, D.; Zuo, H.; Ye, D.; Chen, W.; Zhang, P. Plasma membrane proteomics of tumor spheres identify CD166 as a novel marker for cancer stem-like cells in head and neck squamous cell carcinoma. Mol. Cell. Proteom. 2013, 12, 3271–3284. [Google Scholar] [CrossRef]
- Yang, W.-H.; Lan, H.-Y.; Tai, S.-K.; Yang, M.-H. Repression of bone morphogenetic protein 4 by let-7i attenuates mesenchymal migration of head and neck cancer cells. Biochem. Biophys. Res. Commun. 2013, 433, 24–30. [Google Scholar] [CrossRef]
- Dennis, M.; Wang, G.; Luo, J.; Lin, Y.; Dohadwala, M.; Sidell, D.; DeConde, A.; Abemayor, E.; Elashoff, D.A.; Sharma, S.; et al. Snail controls the mesenchymal phenotype and drives erlotinib resistance in Oral epithelial and HNSCC cells. Otolaryngol. Head Neck Surg. 2012, 147, 726–732. [Google Scholar] [CrossRef]
- Duarte, S.; Loubat, A.; Momier, D.; Topi, M.; Faneca, H.; Pedroso de Lima, M.C.; Carle, G.F.; Pierrefite-Carle, V. Isolation of head and neck squamous carcinoma cancer stem-like cells in a syngeneic mouse model and analysis of hypoxia effect. Oncol. Rep. 2012, 28, 1057–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-S.; Wu, M.-J.; Huang, C.-Y.; Lin, S.-C.; Chuang, T.-H.; Yu, C.-C.; Lo, J.-F. CD133/Src Axis Mediates Tumor Initiating Property and Epithelial-Mesenchymal Transition of Head and Neck Cancer. PLoS ONE 2011, 6, e28053. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Dong, Z.; Vodopyanov, D.; Imai, A.; Helman, J.I.; Prince, M.E.; Wicha, M.S.; Nor, J.E. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010, 70, 9969–9978. [Google Scholar] [CrossRef] [PubMed]
- Kross, K.W.; Heimdal, J.-H.; Olsnes, C.; Olofsson, J.; Aarstad, H.J. Head and neck squamous cell carcinoma spheroid- and monocyte spheroid-stimulated IL-6 and monocyte chemotactic protein-1 secretion are related to TNM stage, inflammatory state and tumor macrophage density. Acta OtoLaryngol. 2005, 125, 1097–1104. [Google Scholar] [CrossRef]
- Kross, K.W.; Heimdal, J.H.; Olsnes, C.; Olofsson, J.; Aarstad, H.J. Co-culture of Head and Neck Squamous Cell Carcinoma Spheroids with Autologous Monocytes Predicts Prognosis. Scand. J. Immunol. 2008, 67, 392–399. [Google Scholar] [CrossRef]
- Harper, L.J.; Piper, K.; Common, J.; Fortune, F.; Mackenzie, I.C. Stem cell patterns in cell lines derived from head and neck squamous cell carcinoma. J. Oral Pathol. Med. 2007, 36, 594–603. [Google Scholar] [CrossRef]
- Olsnes, C.; Heimdal, J.-H.; Kross, K.W.; Olofsson, J.; Aarstad, H.J. Viable head and neck tumor spheroids stimulate in vitro autologous monocyte MCP-1 secretion through soluble substances and CD14/lectin-like receptors. Eur. Arch. Otorhinolaryngol. 2005, 262, 953–960. [Google Scholar] [CrossRef]
- Shan, F.; Close, D.A.; Camarco, D.P.; Johnston, P.A. High-Content Screening Comparison of Cancer Drug Accumulation and Distribution in Two-Dimensional and Three-Dimensional Culture Models of Head and Neck Cancer. Assay Drug Dev. Technol. 2018, 16, 27–50. [Google Scholar] [CrossRef]
- Kellermann, M.G.; Sobral, L.M.; Da Silva, S.D.; Zecchin, K.G.; Graner, E.; Lopes, M.A.; Kowalski, L.P.; Coletta, R.D. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: Induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 2008, 44, 509–517. [Google Scholar] [CrossRef]
- Lewis, M.P.; Lygoe, K.A.; Nystrom, M.L.; Anderson, W.P.; Speight, P.M.; Marshall, J.F.; Thomas, G.J. Tumour-derived TGF-β 1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br. J. Cancer 2004, 90, 822–832. [Google Scholar] [CrossRef]
- Liao, Z.; Tan, Z.W.; Zhu, P.; Tan, N.S. Cancer-associated fibroblasts in tumor microenvironment—Accomplices in tumor malignancy. Cell. Immunol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J. An update on photodynamic therapy applications. J. Clin. Laser Med. Surg. 2002, 20, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Biel, M. Advances in photodynamic therapy for the treatment of head and neck cancers. Lasers Surg. Med. 2006, 38, 349–355. [Google Scholar] [CrossRef]
- Gondivkar, S.M.; Gadbail, A.R.; Choudhary, M.G.; Vedpathak, P.R.; Likhitkar, M.S. Photodynamic treatment outcomes of potentially-malignant lesions and malignancies of the head and neck region: A systematic review. J. Investig. Clin. Dent. 2018, 9. [Google Scholar] [CrossRef]
- Senge, M.O.; Brandt, J.C. Temoporfin (Foscan®, 5, 10, 15, 20-tetra (m-hydroxyphenyl) chlorin)—A second-generation photosensitizer. Photochem. Photobiol. 2011, 87, 1240–1296. [Google Scholar] [CrossRef]
- Yakavets, I.; Millard, M.; Zorin, V.; Lassalle, H.-P.; Bezdetnaya, L. Current state of the nanoscale delivery systems for temoporfin-based photodynamic therapy: Advanced delivery strategies. J. Control. Release 2019, 304, 268–287. [Google Scholar] [CrossRef]
- Mangeolle, T.; Yakavets, I.; Marchal, S.; Debayle, M.; Pons, T.; Bezdetnaya, L.; Marchal, F. Fluorescent Nanoparticles for the Guided Surgery of Ovarian Peritoneal Carcinomatosis. Nanomaterials 2018, 8, 572. [Google Scholar] [CrossRef]
- Egloff-Juras, C.; Bezdetnaya, L.; Dolivet, G.; Lassalle, H.-P. NIR fluorescence-guided tumor surgery: New strategies for the use of indocyanine green. Int. J. Nanomed. 2019, 14, 7823–7838. [Google Scholar] [CrossRef]
- Zhu, B.; Sevick-Muraca, E.M. A review of performance of near-infrared fluorescence imaging devices used in clinical studies. Br. J. Radiol. 2015, 88, 20140547. [Google Scholar] [CrossRef] [Green Version]
- Choromanska, A.; Saczko, J.; Kulbacka, J.; Kamińska, I.; Skolucka, N.; Majkowski, M. Comparison of the influence of photodynamic reaction on the Me45 and MEWO cell lines in vitro. Contemp. Oncol. 2012, 16, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Colley, H.E.; Hearnden, V.; Jones, A.V.; Weinreb, P.H.; Violette, S.M.; Macneil, S.; Thornhill, M.H.; Murdoch, C. Development of tissue-engineered models of oral dysplasia and early invasive oral squamous cell carcinoma. Br. J. Cancer 2011, 105, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Yakavets, I.; Millard, M.; Lamy, L.; Francois, A.; Scheglmann, D.; Wiehe, A.; Lassalle, H.-P.; Zorin, V.; Bezdetnaya, L. Matryoshka-Type Liposomes Offer the Improved Delivery of Temoporfin to Tumor Spheroids. Cancers 2019, 11, 1366. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tao, Y.; Gao, S.; Li, P.; Zheng, J.; Zhang, S.; Liang, J.; Zhang, Y. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine 2018, 36, 209–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, W.B.; Yao, M.; Cheng, N. Priming cancer cells for drug resistance: Role of the fibroblast niche. Front. Biol. 2014, 9, 114–126. [Google Scholar] [CrossRef]
- Young, M.; Rodenhizer, D.; Dean, T.; D’Arcangelo, E.; Xu, B.; Ailles, L.; McGuigan, A.P. A TRACER 3D Co-Culture tumour model for head and neck cancer. Biomaterials 2018, 164, 54–69. [Google Scholar] [CrossRef]
- Thoma, C.R.; Zimmermann, M.; Agarkova, I.; Kelm, J.M.; Krek, W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv. Drug Deliv. Rev. 2014, 69–70, 29–41. [Google Scholar] [CrossRef]
- Bray, L.J.; Hutmacher, D.W.; Bock, N. Addressing Patient Specificity in the Engineering of Tumor Models. Front. Bioeng. Biotechnol. 2019, 7, 217. [Google Scholar] [CrossRef] [Green Version]
- Lazzari, G.; Nicolas, V.; Matsusaki, M.; Akashi, M.; Couvreur, P.; Mura, S. Multicellular spheroid based on a triple co-culture: A novel 3D model to mimic pancreatic tumor complexity. Acta Biomater. 2018, 78, 296–307. [Google Scholar] [CrossRef]
- Bulin, A.-L.; Broekgaarden, M.; Hasan, T. Comprehensive high-throughput image analysis for therapeutic efficacy of architecturally complex heterotypic organoids. Sci. Rep. 2017, 7, 16645. [Google Scholar] [CrossRef]
- Priwitaningrum, D.L.; Blonde, J.-B.G.; Sridhar, A.; Van Baarlen, J.; Hennink, W.E.; Storm, G.; Le Gac, S.; Prakash, J. Tumor stroma-containing 3D spheroid arrays: A tool to study nanoparticle penetration. J. Control. Release 2016, 244, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-A.; Lee, E.K.; Kuh, H.-J. Co-culture of 3D tumor spheroids with fibroblasts as a model for epithelial–mesenchymal transition in vitro. Exp. Cell Res. 2015, 335, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Shoval, H.; Karsch-Bluman, A.; Brill-Karniely, Y.; Stern, T.; Zamir, G.; Hubert, A.; Benny, O. Tumor cells and their crosstalk with endothelial cells in 3D spheroids. Sci. Rep. 2017, 7, 10428. [Google Scholar] [CrossRef] [PubMed]
- Kuen, J.; Darowski, D.; Kluge, T.; Majety, M. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model. PLoS ONE 2017, 12, e0182039. [Google Scholar] [CrossRef]
- Rebelo, S.P.; Pinto, C.; Martins, T.R.; Harrer, N.; Estrada, M.F.; Loza-Alvarez, P.; Cabeçadas, J.; Alves, P.M.; Gualda, E.J.; Sommergruber, W.; et al. 3D-3-culture: A tool to unveil macrophage plasticity in the tumour microenvironment. Biomaterials 2018, 163, 185–197. [Google Scholar] [CrossRef]
- Janina, F.J.F.; Meher, M.M.M. ITOC2—001. Influence of tumour cell—Fibroblast co-culture on monocyte differentiation and tumour growth in pancreatic cancer. Eur. J. Cancer 2015, 51, S1. [Google Scholar] [CrossRef]
- Obaid, G.; Bano, S.; Mallidi, S.; Broekgaarden, M.; Kuriakose, J.; Silber, Z.; Bulin, A.-L.; Wang, Y.; Mai, Z.; Jin, W.; et al. Impacting pancreatic cancer therapy in heterotypic in vitro organoids and in vivo tumors with specificity-tuned, NIR-activable photoimmunonanoconjugates: Towards conquering desmoplasia? Nano Lett. 2019. [Google Scholar] [CrossRef]
- Ware, M.J.; Keshishian, V.; Law, J.J.; Ho, J.C.; Favela, C.A.; Rees, P.; Smith, B.; Mohammad, S.; Hwang, R.F.; Rajapakshe, K.; et al. Generation of an in vitro 3D PDAC stroma rich spheroid model. Biomaterials 2016, 108, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Colley, H.E.; Hearnden, V.; Avila-Olias, M.; Cecchin, D.; Canton, I.; Madsen, J.; MacNeil, S.; Warren, N.; Hu, K.; McKeating, J.A.; et al. Polymersome-Mediated Delivery of Combination Anticancer Therapy to Head and Neck Cancer Cells: 2D and 3D in Vitro Evaluation. Mol. Pharm. 2014, 11, 1176–1188. [Google Scholar] [CrossRef]
- Eke, I.; Leonhardt, F.; Storch, K.; Hehlgans, S.; Cordes, N. The Small Molecule Inhibitor QLT0267 Radiosensitizes Squamous Cell Carcinoma Cells of the Head and Neck. PLoS ONE 2009, 4, e6434. [Google Scholar] [CrossRef]
- Eke, I.; Schneider, L.; Forster, C.; Zips, D.; Kunz-Schughart, L.A.; Cordes, N. EGFR/JIP-4/JNK2 Signaling Attenuates Cetuximab-Mediated Radiosensitization of Squamous Cell Carcinoma Cells. Cancer Res. 2013, 73, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Braunholz, D.; Saki, M.; Niehr, F.; Ozturk, M.; Puertolas, B.B.; Konschak, R.; Budach, V.; Tinhofer, I. Spheroid Culture of Head and Neck Cancer Cells Reveals an Important Role of EGFR Signalling in Anchorage Independent Survival. PLoS ONE 2016, 11, e0163149. [Google Scholar] [CrossRef] [PubMed]
- Kadletz, L.; Heiduschka, G.; Domayer, J.; Schmid, R.; Enzenhofer, E.; Thurnher, D. Evaluation of spheroid head and neck squamous cell carcinoma cell models in comparison to monolayer cultures. Oncol. Lett. 2015, 10, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Khawar, I.A.; Park, J.K.; Jung, E.S.; Lee, M.A.; Chang, S.; Kuh, H.-J. Three Dimensional Mixed-Cell Spheroids Mimic Stroma-Mediated Chemoresistance and Invasive Migration in hepatocellular carcinoma. Neoplasia 2018, 20, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Kawashiri, S.; Tanaka, A.; Noguchi, N.; Hase, T.; Nakaya, H.; Ohara, T.; Kato, K.; Yamamoto, E. Significance of stromal desmoplasia and myofibroblast appearance at the invasive front in squamous cell carcinoma of the oral cavity. Head Neck 2009, 31, 1346–1353. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A.; Knuechel, R. Tumor-associated fibroblasts (part I): Active stromal participants in tumor development and progression? Histol. Histopathol. 2002, 17, 599–621. [Google Scholar]
- Gaio, E.; Scheglmann, D.; Reddi, E.; Moret, F. Uptake and photo-toxicity of Foscan®, Foslip® and Fospeg® in multicellular tumor spheroids. J. Photochem. Photobiol. B 2016, 161, 244–252. [Google Scholar] [CrossRef]
- Mitra, S.; Maugain, E.; Bolotine, L.; Guillemin, F.; Foster, T.H. Temporally and spatially heterogeneous distribution of mTHPC in a murine tumor observed by two-color confocal fluorescence imaging and spectroscopy in a whole-mount model. Photochem. Photobiol. 2005, 81, 1123–1130. [Google Scholar] [CrossRef]
- Yakavets, I.; Yankovsky, I.; Millard, M.; Lamy, L.; Lassalle, H.-P.; Wiehe, A.; Zorin, V.; Bezdetnaya, L. The alteration of temoporfin distribution in multicellular tumor spheroids by β-cyclodextrins. Int. J. Pharm. 2017, 529, 568–575. [Google Scholar] [CrossRef]
- Millard, M.; Yakavets, I.; Piffoux, M.; Brun, A.; Gazeau, F.; Guigner, J.-M.; Jasniewski, J.; Lassalle, H.-P.; Wilhelm, C.; Bezdetnaya, L. mTHPC-loaded extracellular vesicles outperform liposomal and free mTHPC formulations by an increased stability, drug delivery efficiency and cytotoxic effect in tridimensional model of tumors. Drug Deliv. 2018, 25, 1790–1801. [Google Scholar] [CrossRef] [Green Version]
- Farrakhova, D.S.; Yakavets, I.V.; Loschenov, V.B.; Bolotine, L.N.; Zorin, V.P. Investigation of chlorin photosensitizers distribution in monolayer and spheroid cell cultures. Biomed. Photonics 2017, 6, 4–11. [Google Scholar] [CrossRef]
- Kumari, P.; Jain, S.; Ghosh, B.; Zorin, V.; Biswas, S. Polylactide-Based Block Copolymeric Micelles Loaded with Chlorin e6 for Photodynamic Therapy: In Vitro Evaluation in Monolayer and 3D Spheroid Models. Mol. Pharm. 2017, 14, 3789–3800. [Google Scholar] [CrossRef] [PubMed]
- Farrakhova, D.; Maklygina, Y.; Romanishkin, I.; Ryabova, A.; Yakavets, I.; Millard, M.; Bolotine, L.; Plyutinskaya, A.; Karmakova, T.; Pankratov, A.; et al. Investigation of Ce6 accumulation and distribution in cell cultures of head and neck cancers. In Proceedings of the Saratov Fall Meeting 2018: Optical and Nano-Technologies for Biology and Medicine, Bellingham, WA, USA, 24–28 September 2018; International Society for Optics and Photonics: Bellingham, WA, USA, 2019; Volume 11065, p. 110651V. [Google Scholar]
- Tanaka, H.Y.; Kano, M.R. Stromal barriers to nanomedicine penetration in the pancreatic tumor microenvironment. Cancer Sci. 2018, 109, 2085–2092. [Google Scholar] [CrossRef] [PubMed]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakavets, I.; Jenard, S.; Francois, A.; Maklygina, Y.; Loschenov, V.; Lassalle, H.-P.; Dolivet, G.; Bezdetnaya, L. Stroma-Rich Co-Culture Multicellular Tumor Spheroids as a Tool for Photoactive Drugs Screening. J. Clin. Med. 2019, 8, 1686. https://doi.org/10.3390/jcm8101686
Yakavets I, Jenard S, Francois A, Maklygina Y, Loschenov V, Lassalle H-P, Dolivet G, Bezdetnaya L. Stroma-Rich Co-Culture Multicellular Tumor Spheroids as a Tool for Photoactive Drugs Screening. Journal of Clinical Medicine. 2019; 8(10):1686. https://doi.org/10.3390/jcm8101686
Chicago/Turabian StyleYakavets, Ilya, Samuel Jenard, Aurelie Francois, Yulia Maklygina, Victor Loschenov, Henri-Pierre Lassalle, Gilles Dolivet, and Lina Bezdetnaya. 2019. "Stroma-Rich Co-Culture Multicellular Tumor Spheroids as a Tool for Photoactive Drugs Screening" Journal of Clinical Medicine 8, no. 10: 1686. https://doi.org/10.3390/jcm8101686
APA StyleYakavets, I., Jenard, S., Francois, A., Maklygina, Y., Loschenov, V., Lassalle, H. -P., Dolivet, G., & Bezdetnaya, L. (2019). Stroma-Rich Co-Culture Multicellular Tumor Spheroids as a Tool for Photoactive Drugs Screening. Journal of Clinical Medicine, 8(10), 1686. https://doi.org/10.3390/jcm8101686