Histopathological Verification of the Diagnostic Performance of the EU-TIRADS Classification of Thyroid Nodules—Results of a Multicenter Study Performed in a Previously Iodine-Deficient Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ultrasound Examination
2.3. EU-TIRADS Score
- EU-TIRADS 2 (benign category): purely cystic, entirely spongiform; risk of malignancy (RM): close to 0%, without a recommendation for biopsy.
- EU-TIRADS 3 (low-risk category): ovoid, smooth isoechoic/hyperechoic, no highly suspicious characteristics; RM: 2%–4%, recommendation for biopsy only for nodules >20 mm.
- EU-TIRADS 4 (intermediate-risk category): ovoid, smooth, mildly hypoechoic, no highly suspicious characteristics; RM: 6%–17%, recommendation for biopsy usually for nodules >15 mm.
- EU-TIRADS 5 (high-risk category): at least 1 of the following highly suspicious characteristics: irregular shape (taller-than-wide shape), irregular margins, microcalcifications, marked hypoechogenicity (and solid); RM: 26%–87%, recommendation for biopsy for nodules >10 mm.
2.4. Histopathological Examination
2.5. Statistical Analysis
2.6. Ethical Approval
2.7. Informed Consent
3. Results
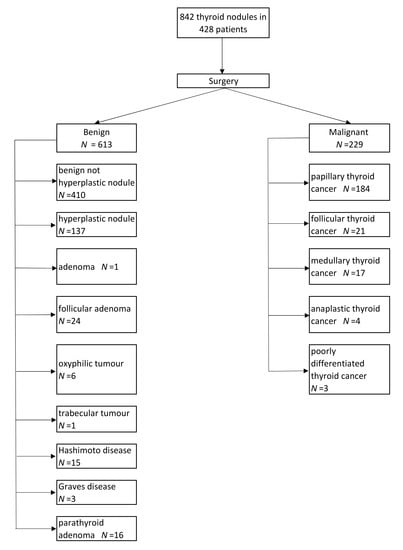
3.1. Patients
3.2. Histopathological Examination
3.3. Conventional B-Mode Ultrasound Examination and EU-TIRADS Categorization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahn, H.S.; Kim, H.J.; Welch, H.G. Korea’s thyroid-cancer “epidemic”—Screening and overdiagnosis. N. Engl. J. Med. 2014, 371, 1765–1767. [Google Scholar] [CrossRef] [PubMed]
- Reiners, C.; Wegscheider, K.; Schicha, H.; Theissen, P.; Vaupel, R.; Wrbitzky, R.; Schumm-Draeger, P.M. Prevalence of thyroid disorders in the working population of Germany: Ultrasonography screening in 96,278 unselected employees. Thyroid 2004, 14, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Wolinski, K.; Szczepanek-Parulska, E.; Stangierski, A.; Gurgul, E.; Rewaj-Losyk, M.; Ruchala, M. How to select nodules for fine-needle aspiration biopsy in multinodular goitre. Role of conventional ultrasonography and shear wave elastography—A preliminary study. Endokrynol. Pol. 2014, 65, 114–118. [Google Scholar] [PubMed]
- Burch, H.B.; Burman, K.D.; Cooper, D.S.; Hennessey, J.V.; Vietor, N.O. A 2015 survey of clinical practice patterns in the management of thyroid nodules. J. Clin. Endocrinol. Metab. 2016, 101, 2853–2862. [Google Scholar] [CrossRef] [PubMed]
- Kwong, N.; Medici, M.; Angell, T.E.; Liu, X.; Marqusee, E.; Cibas, E.S.; Krane, J.F.; Barletta, J.A.; Kim, M.I.; Larsen, P.R.; et al. The influence of patient age on thyroid nodule formation, multinodularity, and thyroid cancer risk. J. Clin. Endocrinol. Metab. 2015, 100, 4434–4440. [Google Scholar] [CrossRef]
- Szczepanek-Parulska, E.; Wolinski, K.; Stangierski, A.; Gurgul, E.; Biczysko, M.; Majewski, P.; Rewaj-Losyk, M.; Ruchala, M. Comparison of diagnostic value of conventional ultrasonography and shear wave elastography in the prediction of thyroid lesions malignancy. PLoS ONE 2013, 8, e81532. [Google Scholar] [CrossRef]
- Adamczewski, Z.; Lewinski, A. Proposed algorithm for management of patients with thyroid nodules/focal lesions, based on ultrasound (US) and fine-needle aspiration biopsy (FNAB); Our own experience. Thyroid Res. 2013, 6, 6. [Google Scholar] [CrossRef]
- Kang, H.W.; No, J.H.; Chung, J.H.; Min, Y.K.; Lee, M.S.; Lee, M.K.; Yang, J.H.; Kim, K.W. Prevalence, clinical and ultrasonographic characteristics of thyroid incidentalomas. Thyroid 2004, 14, 29–33. [Google Scholar] [CrossRef]
- Witczak, J.; Taylor, P.; Chai, J.; Amphlett, B.; Soukias, J.M.; Das, G.; Tennant, B.P.; Geen, J.; Okosieme, O.E. Predicting malignancy in thyroid nodules: Feasibility of a predictive model integrating clinical, biochemical, and ultrasound characteristics. Thyroid Res. 2016, 9, 4. [Google Scholar] [CrossRef]
- Horvath, E.; Majlis, S.; Rossi, R.; Franco, C.; Niedmann, J.P.; Castro, A.; Dominguez, M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J. Clin. Endocrinol. Metab. 2009, 94, 1748–1751. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Jarzab, B.; Dedecjus, M.; Slowinska-Klencka, D.; Lewinski, A.; Adamczewski, Z.; Anielski, R.; Baglaj, M.; Baldys-Waligorska, A.; Barczynski, M.; Bednarczuk, T.; et al. Guidelines of Polish National Societies diagnostics and treatment of thyroid carcinoma. 2018 update. Endokrynol. Pol. 2018, 69, 34–74. [Google Scholar] [PubMed]
- Lauria Pantano, A.; Maddaloni, E.; Briganti, S.I.; Beretta Anguissola, G.; Perrella, E.; Taffon, C.; Palermo, A.; Pozzilli, P.; Manfrini, S.; Crescenzi, A. Differences between ATA, AACE/ACE/AME and ACR TI-RADS ultrasound classifications performance in identifying cytological high-risk thyroid nodules. Eur. J. Endocrinol. 2018, 178, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Ngu, R.; Royer, B.; Giovanella, L.; Bigorgne, C.; Simo, R.; Carroll, P.; Russ, G. A multicentre validation study for the EU-TIRADS using histological diagnosis as a gold standard. Clin. Endocrinol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Takkouche, B.; Egli, I.; Allen, H.E.; de Benoist, B. Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull. World Health Organ. 2005, 83, 518–525. [Google Scholar] [PubMed]
- Bath, S.C.; Combet, E.; Scully, P.; Zimmermann, M.B.; Hampshire-Jones, K.H.; Rayman, M.P. A multi-centre pilot study of iodine status in UK schoolchildren, aged 8–10 years. Eur. J. Nutr. 2016, 55, 2001–2009. [Google Scholar] [CrossRef]
- Szybinski, Z.; Polish Council for Control of Iodine Deficiency Disorders. Work of the Polish Council for control of Iodine Deficiency Disorders, and the model of iodine prophylaxis in Poland. Endokrynol. Pol. 2012, 63, 156–160. [Google Scholar]
- Gietka-Czernel, M.; Debska, M.; Kretowicz, P.; Jastrzebska, H.; Kondracka, A.; Snochowska, H.; Oltarzewski, M. Iodine status of pregnant women from central Poland ten years after introduction of iodine prophylaxis programme. Endokrynol. Pol. 2010, 61, 646–651. [Google Scholar]
- Gietka-Czernel, M. The thyroid gland in postmenopausal women: Physiology and diseases. Prz. Menopauzalny 2017, 16, 33–37. [Google Scholar] [CrossRef]
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedus, L.; Paschke, R.; Valcavi, R.; Vitti, P. American association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules—2016 update. Endocr. Pract. 2016, 22, 622–639. [Google Scholar] [CrossRef]
- Dobruch-Sobczak, K.S.; Krauze, A.; Migda, B.; Mlosek, K.; Slapa, R.Z.; Bakula-Zalewska, E.; Adamczewski, Z.; Lewinski, A.; Jakubowski, W.; Dedecjus, M. Integration of sonoelastography into the tirads lexicon could influence the classification. Front. Endocrinol. 2019, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Wolinski, K.; Szkudlarek, M.; Szczepanek-Parulska, E.; Ruchala, M. Usefulness of different ultrasound features of malignancy in predicting the type of thyroid lesions: A meta-analysis of prospective studies. Pol. Arch. Med. Wewn 2014, 124, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, V.; Maceroni, P.; D’Andrea, V.; Patrizi, G.; Di Segni, M.; De Vito, C.; Grazhdani, H.; Isidori, A.M.; Giannetta, E.; Redler, A.; et al. Strain ratio ultrasound elastography increases the accuracy of colour-Doppler ultrasound in the evaluation of Thy-3 nodules. A bi-centre university experience. Eur. Radiol. 2016, 26, 1441–1449. [Google Scholar]
- Middleton, W.D.; Teefey, S.A.; Reading, C.C.; Langer, J.E.; Beland, M.D.; Szabunio, M.M.; Desser, T.S. Comparison of performance characteristics of American College of Radiology TI-RADS, Korean Society of Thyroid Radiology TIRADS, and American Thyroid Association guidelines. Am. J. Roentgenol. 2018, 210, 1148–1154. [Google Scholar] [CrossRef]
- Ha, E.J.; Na, D.G.; Moon, W.J.; Lee, Y.H.; Choi, N. Diagnostic performance of ultrasound-based risk-stratification systems for thyroid nodules: Comparison of the 2015 American Thyroid Association guidelines with the 2016 Korean Thyroid Association/Korean Society of Thyroid Radiology and 2017 American College of Radiology guidelines. Thyroid 2018, 28, 1532–1537. [Google Scholar]
- Phuttharak, W.; Boonrod, A.; Klungboonkrong, V.; Witsawapaisan, T. Interrater reliability of various thyroid imaging reporting and data system (TIRADS) classifications for differentiating benign from malignant thyroid nodules. Asian Pac. J. Cancer Prev. 2019, 20, 1283–1288. [Google Scholar] [CrossRef]
- Skowronska, A.; Milczarek-Banach, J.; Wiechno, W.; Chudzinski, W.; Zach, M.; Mazurkiewicz, M.; Miskiewicz, P.; Bednarczuk, T. Accuracy of the European thyroid imaging reporting and data system (EU-TIRADS) in the valuation of thyroid nodule malignancy in reference to the post-surgery histological results. Pol. J. Radiol. 2018, 83, e579–e586. [Google Scholar] [CrossRef]
- Zhang, T.T.; Li, C.F.; Wen, S.S.; Huang, D.Z.; Sun, G.H.; Zhu, Y.X.; Wang, Y.; Ji, Q.H.; Qu, N.; Shi, R.L. Effects of tumor size on prognosis in differentiated thyroid carcinoma smaller than 2 cm. Oncol. Lett. 2019, 17, 4229–4236. [Google Scholar] [CrossRef]
- Sun, W.; Lan, X.; Zhang, H.; Dong, W.; Wang, Z.; He, L.; Zhang, T.; Liu, S. Risk factors for central lymph node metastasis in CN0 papillary thyroid carcinoma: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0139021. [Google Scholar] [CrossRef]
- Lewinski, A.; Adamczewski, Z. Papillary thyroid carcinoma: A cancer with an extremely diverse genetic background and prognosis. Pol. Arch. Intern. Med. 2017, 127, 388–389. [Google Scholar] [CrossRef]
- Trimboli, P.; Piccardo, A.; Alevizaki, M.; Virili, C.; Naseri, M.; Sola, S.; Paone, G.; Russ, G.; Giovanella, L. Dedicated neck 18F-FDG PET/CT: An additional tool for risk assessment in thyroid nodules at ultrasound intermediate risk. Clin. Endocrinol. 2019, 90, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Paone, G.; Treglia, G.; Virili, C.; Ruberto, T.; Ceriani, L.; Piccardo, A.; Giovanella, L. Fine-needle aspiration in all thyroid incidentalomas at 18F-FDG PET/CT: Can EU-TIRADS revise the dogma? Clin. Endocrinol. 2018, 89, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Borowczyk, M.; Szczepanek-Parulska, E.; Olejarz, M.; Więckowska, B.; Verburg, F.A.; Dębicki, S.; Budny, B.; Janicka-Jedyńska, M.; Ziemnicka, K.; Ruchała, M. Evaluation of 167 Gene Expression Classifier (GEC) and ThyroSeq v2 diagnostic accuracy in the preoperative assessment of indeterminate thyroid nodules: Bivariate/HROC meta-analysis. Endocr. Pathol. 2019, 30, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zolotov, S. Genetic testing in differentiated thyroid carcinoma: Indications and clinical implications. Rambam Maimonides Med. J. 2016, 28, 7. [Google Scholar] [CrossRef]


| Nodules | Patients | |
|---|---|---|
| Number of nodules found per patient | 1 | 231 |
| 2 | 112 | |
| 3 | 24 | |
| 4 | 26 | |
| 5 | 14 | |
| 6 | 9 | |
| 7 | 9 | |
| 8 | 3 | |
| In total | 842 | 428 |
| EU-TIRADS Category | Benign Lesions (%) | Malignant Lesions (%) |
|---|---|---|
| 2 | 154 (100) | - |
| 3 | 90 (97) | 3 (3) |
| 4 | 91 (81) | 12 (19) |
| 5 | 278 (57) | 214 (43) |
| Ultrasound Feature | Characteristics% (95% CI) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | OR |
|---|---|---|---|---|---|---|
| Echogenicity | Markedly hypoechoic | 59.0 (52.3–65.4) | 71.8 (68.0–75.3) | 43.8 (38.2–49.6) | 82.4 (78.9–85.5) | 3.65 (2.66–5.01) |
| Hypoechoic | 93.9 (90.0–96.6) | 27.2 (23.8–31.0) | 32.5 (29.0–36.2) | 92.3 (87.4–95.7) | 5.75 (3.25–10.16) | |
| Isoechoic | 5.2 (2.7–9.0) | 76.2 (72.6–79.5) | 7.6 (4.0–12.9) | 68.3 (64.6–71.8) | 0.18 (0.10–0.33) | |
| Hyperechoic | 0.9 (0.1–3.1) | 96.9 (95.2–98.1) | 9.5 (1.2–30.4) | 72.4 (69.2–75.4) | 0.28 (0.06–1.19) | |
| Margins | Irregular | 75.5 (69.5–81.0) | 81.7 (78.4–84.7) | 60.7 (54.8–66.4) | 89.9 (87.1–92.3) | 13.82 (9.6–19.9) |
| Microcalcifications | Yes | 53.7 (47.0–60.3) | 75.9 (72.3–79.2) | 45.4 (39.4–51.5) | 81.4 (78.0–84.5) | 3.65 (2.65–5.02) |
| Macrocalcifications | Yes | 22.3 (17.1–28.2) | 84.8 (81.7–87.6) | 35.4 (27.6–43.8) | 74.5 (71.1–77.7) | 1.60 (1.09–2.35) |
| Composition | Solid/Almost solid | 92.6 (88.4–95.6) | 44.0 (40.1–48.1) | 38.2 (34.1–42.4) | 94.1 (90.7–96.5) | 9.82 (5.84–16.50) |
| Solid-cystic | 7.4 (4.4–11.6) | 59.7 (55.7–63.6) | 6.4 (3.8–10.1) | 63.3 (59.2–67.3) | 0.12 (0.07–0.20) | |
| Cystic | 0.0 (0.0–1.6) | 99.7 (98.8–100.0) | 0.0 (0.0–84.2) | 72.7 (69.6–75.7) | NA | |
| Spongiform | 0.0 (0.0–1.6) | 96.6 (94.8–97.9) | 0.0 (0.0–16.1) | 72.1 (68.9–75.2) | NA | |
| Shape | Taller-than-wide shape | 45.9 (39.3–52.5) | 85.2 (82.1–87.9) | 53.6 (46.3–60.7) | 80.8 (77.6–83.8) | 4.86 (3.45–6.84) |
| EU-TIRADS Score | Positive | Negative | Value | CI (95%) | ||
|---|---|---|---|---|---|---|
| ≥3 | Malignant | 229 | 0 | Sensitivity (%) | 100.0 | 98.4–100.0 |
| Benign | 459 | 154 | Specificity (%) | 25.1 | 21.7–28.8 | |
| Accuracy (%) | 45.5 | 42.1–48.9 | ||||
| PPV (%) | 33.3 | 29.8–36.9 | ||||
| NPV (%) | 100 | 97.6–100.0 | ||||
| OR | NA | NA | ||||
| ≥4 | Malignant | 226 | 3 | Sensitivity (%) | 98.7 | 96.2–99.7 |
| Benign | 369 | 244 | Specificity (%) | 39.8 | 35.9–43.8 | |
| Accuracy (%) | 55.8 | 52.4–59.2 | ||||
| PPV (%) | 38 | 34.1–42.0 | ||||
| NPV (%) | 98.8 | 96.5–99.7 | ||||
| OR | 49.8 | 15.8–157.4 | ||||
| 5 | Malignant | 214 | 15 | Sensitivity (%) | 93.4 | 89.4–96.3 |
| Benign | 278 | 335 | Specificity (%) | 54.6 | 50.6–58.6 | |
| Accuracy (%) | 65.2 | 61.9–68.4 | ||||
| PPV (%) | 43.5 | 39.1–48.0 | ||||
| NPV (%) | 95.7 | 93–97.6 | ||||
| OR | 17.2 | 9.95–29.71 | ||||
| EU- TIRADS Score | Diameter (mm) | Benign | Malignant | Accuracy (%) (95% CI) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| 3 | ≤20 | 53 | 2 | 58.1 (47.4–68.2) | 33.3 (0.8–90.6) | 58.9 (48–69.2) | 2.6 (0.1–13.8) | 96.4 (87.5–99.6) | 0.72 (0.06–8.19) |
| >20 | 37 | 1 | |||||||
| ≤15 | 38 | 2 | 41.9 (31.8–52.6) | 33.3 (0.8–90.6) | 42.2 (31.9–53.1) | 1.9 (0–10.1) | 95.0 (83.1–99.4) | 0.37 (0.03–4.18) | |
| >15 | 52 | 1 | |||||||
| ≤10 | 17 | 1 | 20.4 (12.8–30.1) | 66.7 (9.4–99.2) | 18.9 (11.4–28.5) | 2.7 (0.3–9.3) | 94.4 (72.7–99.9) | 0.47 (0.04–5.44) | |
| >10 | 73 | 2 | |||||||
| ≤5 | 0 | 0 | 3.2 (0.67–9.14) | 100 (29.24–100.0) | 0 (0.0–4.02) | 3.2 (0.67–9.14) | NA | NA | |
| >5 | 90 | 3 | |||||||
| 4 | ≤15 | 46 | 7 | 49.5 (39.5–59.5) | 41.7 (15.2–72.3) | 50.5 (39.9–61.2) | 10.0 (3.3–21.8) | 86.8 (74.7–94.5) | 0.73 (0.22–2.47) |
| >15 | 45 | 5 | |||||||
| ≤10 | 19 | 5 | 25.2 (17.2–34.8) | 58.3 (27.7–84.8) | 20.9 (13.1–30.7) | 8.9 (3.6–17.4) | 79.2 (57.8–92.9) | 0.37 (0.11–1.29) | |
| >10 | 72 | 7 | |||||||
| ≤5 | 0 | 1 | 10.7 (5.5–18.3) | 91.7 (61.5–99.8) | 0.0 (0.0–4.0) | 10.8 (5.5–18.5) | 0.0 (0.0–97.5) | NA | |
| >5 | 91 | 11 | |||||||
| 5 | ≤10 | 82 | 72 | 45.5 (41.1–50.0) | 66.4 (59.6–72.7) | 29.5 (24.2–35.2) | 42.0 (36.7–47.5) | 53.2 (45–61.3) | 0.83 (0.56–1.21) |
| >10 | 196 | 142 | |||||||
| ≤5 | 8 | 6 | 43.9 (39.5–48.4) | 97.2 (94.0–99.0) | 2.9 (1.3–5.6) | 43.5 (39.0–48.1) | 57.1 (28.9–82.3) | 1.03 (0.35–3.01) | |
| >5 | 270 | 208 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobruch-Sobczak, K.; Adamczewski, Z.; Szczepanek-Parulska, E.; Migda, B.; Woliński, K.; Krauze, A.; Prostko, P.; Ruchała, M.; Lewiński, A.; Jakubowski, W.; et al. Histopathological Verification of the Diagnostic Performance of the EU-TIRADS Classification of Thyroid Nodules—Results of a Multicenter Study Performed in a Previously Iodine-Deficient Region. J. Clin. Med. 2019, 8, 1781. https://doi.org/10.3390/jcm8111781
Dobruch-Sobczak K, Adamczewski Z, Szczepanek-Parulska E, Migda B, Woliński K, Krauze A, Prostko P, Ruchała M, Lewiński A, Jakubowski W, et al. Histopathological Verification of the Diagnostic Performance of the EU-TIRADS Classification of Thyroid Nodules—Results of a Multicenter Study Performed in a Previously Iodine-Deficient Region. Journal of Clinical Medicine. 2019; 8(11):1781. https://doi.org/10.3390/jcm8111781
Chicago/Turabian StyleDobruch-Sobczak, Katarzyna, Zbigniew Adamczewski, Ewelina Szczepanek-Parulska, Bartosz Migda, Kosma Woliński, Agnieszka Krauze, Piotr Prostko, Marek Ruchała, Andrzej Lewiński, Wiesław Jakubowski, and et al. 2019. "Histopathological Verification of the Diagnostic Performance of the EU-TIRADS Classification of Thyroid Nodules—Results of a Multicenter Study Performed in a Previously Iodine-Deficient Region" Journal of Clinical Medicine 8, no. 11: 1781. https://doi.org/10.3390/jcm8111781
APA StyleDobruch-Sobczak, K., Adamczewski, Z., Szczepanek-Parulska, E., Migda, B., Woliński, K., Krauze, A., Prostko, P., Ruchała, M., Lewiński, A., Jakubowski, W., & Dedecjus, M. (2019). Histopathological Verification of the Diagnostic Performance of the EU-TIRADS Classification of Thyroid Nodules—Results of a Multicenter Study Performed in a Previously Iodine-Deficient Region. Journal of Clinical Medicine, 8(11), 1781. https://doi.org/10.3390/jcm8111781