Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces
Abstract
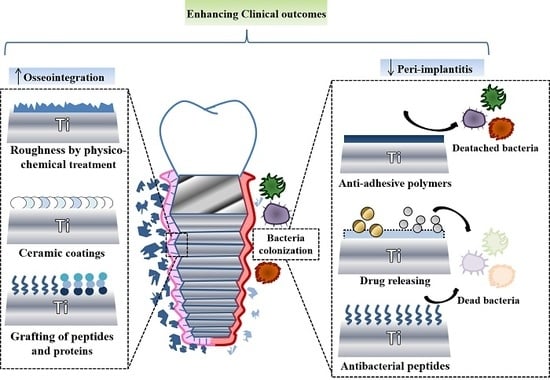
:1. Introduction
2. Current Osteogenic Strategies in Implantology
2.1. Macro and Microroughness Surfaces
2.2. Nanoroughness Surfaces
2.3. Inorganic Coatings
2.4. Bacteria Colonization
3. Current Trends: Osteogenic Coatings with Antibacterial Potential
3.1. Metal Ions and Nanoparticles
3.2. Bactericidal Peptides
3.3. Antibiotics
3.4. Anti-Adhesive Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Res. 2014, 40, 50–60. [Google Scholar] [CrossRef]
- Duraccio, D.; Mussano, F.; Faga, M.G. Biomaterials for dental implants: Current and future trends. J. Mater. Sci. Mater. Med. 2015, 50, 4779–4812. [Google Scholar] [CrossRef]
- Smith, D.C. Dental implants: Materials and design considerations. Int. J. Prosthodont. 1993, 6, 106–117. [Google Scholar]
- Kasemo, B. Biocompatibility of titanium implants: Surface science aspects. J. Prosthet. Dent. 1983, 49, 832–837. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface modifications and their effects on titanium dental implants. BioMed Res. Int. 2015. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Pedrosa, A.R.; Martins, M.D. Chemical and topographic analysis of treated surfaces of five different commercial dental titanium implants. Mat. Res. 2012, 15, 372–382. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials science: An evolving, multidisciplinary endeavor. Available online: https://www.researchgate.net/publication/288163931_Biomaterials_Science_An_Evolving_Multidisciplinary_Endeavor (accessed on 31 December 2013).
- Snauwaert, K.; Duyck, J.; van Steenberghe, D.; Quirynen, M.; Naert, I. Time dependent failure rate and marginal bone loss of implant supported prostheses: A 15-year follow-up study. Clin. Oral Investig. 2000, 4, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sumner, D.R.; Galante, J.O. Determinants of stress shielding. Clin. Orthop. Relat. Res. 1991, 274, 202–212. [Google Scholar] [CrossRef]
- Huiskes, R.; Weinans, H.; Van Rietbergen, B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Available online: https://repub.eur.nl/pub/15376 (accessed on 1 January 1992).
- Tengvall, P.; Textor, M.; Thomsen, P. (Eds.) Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Springer-Verlag Berlin Heidelberg: Heidelberg, Germany, 2012. [Google Scholar]
- Torres, Y.; Trueba, P.; Pavón, J.; Montealegre, I.; Rodríguez-Ortiz, J. Designing, processing and characterisation of titanium cylinders with graded porosity: An alternative to stress-shielding solutions. Mater. Des. 2014, 63, 316–324. [Google Scholar] [CrossRef]
- Mombelli, A.; Lang, N.P. The diagnosis and treatment of peri-implantitis. Periodontology 1998, 17, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Katsikogianni, M.; Missirlis, Y. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cell Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial coatings: Challenges, perspectives, and opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef]
- Rompen, E.; Domken, O.; Degidi, M.; Farias Pontes, A.E.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implants Res. 2006, 17, 55–67. [Google Scholar] [CrossRef]
- Yamano, S.; Al-Sowygh, Z.H.; Gallucci, G.O.; Wada, K.; Weber, H.P.; Sukotjo, C. Early peri-implant tissue reactions on different titanium surface topographies. Clin. Oral Implants Res. 2011, 22, 815–819. [Google Scholar] [CrossRef]
- Neuss, S.; Schneider, R.K.; Tietze, L.; Knüchel, R.; Jahnen-Dechent, W. Secretion of fibrinolytic enzymes facilitates human mesenchymal stem cell invasion into fibrin clots. Cells Tissues Organs 2010, 191, 36–46. [Google Scholar] [CrossRef]
- Davies, J. Mechanisms of endosseous integration. Int. J. Prosthodont. 1998, 11, 391–401. [Google Scholar]
- Donos, N.; Hamlet, S.; Lang, N.P.; Salvi, G.; Huynh-Ba, G.; Bosshardt, D.; Ivanovski, S. Gene expression profile of osseointegration of a hydrophilic compared with a hydrophobic microrough implant surface. Clin. Oral Implants Res. 2011, 22, 365–372. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implants Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Schwarz, F.; Herten, M.; Sager, M.; Wieland, M.; Dard, M.; Becker, J. Histological and immunohistochemical analysis of initial and early osseous integration at chemically modified and conventional SLA® titanium implants: Preliminary results of a pilot study in dogs. Clin. Oral Implants Res. 2007, 18, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Herten, M.; Wieland, M.; Dard, M.; Becker, J. Chemically modified, ultra-hydrophilic titanium implant surfaces. Mund Kiefer Gesichtschir. 2007, 11, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Scheideler, L.; Eichler, M.; Geis-Gerstorfer, J. Wetting behavior of dental implants. Int. J. Oral Maxillofac. Implants 2011, 26, 1256–1266. [Google Scholar] [PubMed]
- Rupp, F.; Scheideler, L.; Rehbein, D.; Axmann, D.; Geis-Gerstorfer, J. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials 2004, 25, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Scheideler, L.; Olshanska, N.; De Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res. 2006, 76, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Long, M.W.; Robinson, J.; Ashcraft, E.; Mann, K.G. Regulation of human bone marrow-derived osteoprogenitor cells by osteogenic growth factors. J. Clin. Investig. 1995, 95, 881–887. [Google Scholar] [CrossRef]
- Lange, R.; Lüthen, F.; Beck, U.; Rychly, J.; Baumann, A.; Nebe, B. Cell-extracellular matrix interaction and physico-chemical characteristics of titanium surfaces depend on the roughness of the material. Biomol. Eng. 2002, 19, 255–261. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T.; Andersson, B.; Krol, J. A histomorghometric study of screw-shaped and removal torque titanium implants with three different surface topographies. Clin. Oral Implants Res. 1995, 6, 24–30. [Google Scholar] [CrossRef]
- Becker, W.; Becker, B.E.; Ricci, A.; Bahat, O.; Rosenberg, E.; Rose, L.F.; Handelsman, M.; Israelson, H. A prospective multicenter clinical trial comparing one-and two-stage titanium screw-shaped fixtures with one-stage plasma-sprayed solid-screw fixtures. Clin. Implant Dent. Relat. Res. 2000, 2, 159–165. [Google Scholar] [CrossRef]
- Leimola-Virtanen, R.; Peltola, J.; Oksala, E.; Helenius, H.; Happonen, R.-P. ITI titanium plasma-sprayed screw implants in the treatment of edentulous mandibles: A follow-up study of 39 patients. Int. J. Oral Maxillofac. Implants 1995, 10, 56–58. [Google Scholar] [CrossRef]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- De Groot, K.; Wolke, J.; Jansen, J. Calcium phosphate coatings for medical implants. Proc. Inst. Mech. Eng. H 1998, 212, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [PubMed]
- Huang, H.; Manga, Y.; Huang, W.-N.; Lin, C.-K.; Tseng, C.-L.; Huang, H.-M.; Wu, C.-Y.; Wu, C.-C. Effect of hydroxyapatite formation on titanium surface with bone morphogenetic protein-2 loading through electrochemical deposition on MG-63 cells. Mater. Des. 2018, 11, 1897. [Google Scholar] [CrossRef] [Green Version]
- Geurs, N.C.; Jeffcoat, R.L.; McGlumphy, E.A.; Reddy, M.S.; Jeffcoat, M.K. Influence of implant geometry and surface characteristics on progressive osseointegration. Int. J. Oral Maxillofac. Implants 2002, 17, 811–815. [Google Scholar]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface treatments and functional coatings for biocompatibility improvement and bacterial adhesion reduction in dental implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Lausmaa, J. Mechanical, thermal, chemical and electrochemical surface treatment of titanium. In Titanium in Medicine; Brunette, D.M., Tengvall, P., Textor, M., Thomsen, P., Eds.; Springer-Verlag Berlin Heidelberg: Heidelberg, Germany, 2001; pp. 231–266. [Google Scholar]
- Larry, L.H. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar]
- Kaur, G.; Sharma, P.; Kumar, V.; Singh, K. Assessment of in vitro bioactivity of SiO2-BaO-ZnO-B2O3-Al2O3 glasses: An optico-analytical approach. Mater. Sci. Eng. C 2012, 32, 1941–1947. [Google Scholar] [CrossRef]
- Yurttutan, M.E.; Keskin, A. Evaluation of the effects of different sand particles that used in dental implant roughened for osseointegration. BMC Oral Health 2018, 18, 47. [Google Scholar] [CrossRef] [Green Version]
- Donati, M.; Ekestubbe, A.; Lindhe, J.; Wennstrom, J.L. Implant-supported single-tooth restorations. A 12-year prospective study. Clin. Oral Implants Res. 2016, 27, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Al-Nawas, B.; Kammerer, P.W.; Morbach, T.; Ladwein, C.; Wegener, J.; Wagner, W. Ten-Year Retrospective Follow-Up Study of the TiOblast (TM) Dental Implant. Clin. Implant. Dent. Relat. Res. 2012, 14, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Donati, M.; Ekestubbe, A.; Lindhe, J.; Wennstrom, J.L. Marginal bone loss at implants with different surface characteristics—A 20-year follow-up of a randomized controlled clinical trial. Clin. Oral Implants Res. 2018, 29, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Ravald, N.; Dahlgren, S.; Teiwik, A.; Gröndahl, K. Long-term evaluation of A stra T ech and B rånemark implants in patients treated with full-arch bridges. Results after 12–15 years. Clin. Oral Implants Res. 2013, 24, 1144–1151. [Google Scholar] [CrossRef]
- Mangano, F.G.; Pires, J.T.; Shibli, J.A.; Mijiritsky, E.; Iezzi, G.; Piattelli, A.; Mangano, C. Early bone response to dual acid-etched and machined dental implants placed in the posterior maxilla: A histologic and histomorphometric human study. Implant Dent. 2017, 26, 24–29. [Google Scholar] [CrossRef]
- Sullivan, D.; Sherwood, R.; Porter, S. Long-term performance of Osseotite implants: A 6-year clinical follow-up. Compend. Contin. Educ. Dent. 2001, 22, 326–328. [Google Scholar]
- Testori, T.; Wiseman, L.; Woolfe, S.; Porter, S.S. A prospective multicenter clinical study of the Osseotite implant: Four-year interim report. Int. J. Oral Maxillofac. Implants 2001, 16, 193–200. [Google Scholar]
- Feldman, S.; Boitel, N.; Weng, D.; Kohles, S.S.; Stach, R.M. Five-Year Survival Distributions of Short-Length (10 mm or less) Machined-Surfaced and Osseotite® Implants. Clin. Implant Dent. Relat. Res. 2004, 6, 16–23. [Google Scholar] [CrossRef]
- Klokkevold, P.R.; Nishimura, R.D.; Adachi, M.; Caputo, A. Osseointegration enhanced by chemical etching of the titanium surface. A torque removal study in the rabbit. Clin. Oral Implants Res. 1997, 8, 442–447. [Google Scholar] [CrossRef]
- Camarda, A.J.; Milot, P.; Ciaburro, H.; Rompré, P.H.; Sallaleh, I.; Alexandre, C.M. Long-term randomized clinical trial evaluating the effects of fixture surface acid-etching and machined collar design on bone healing. Quintessence Int. 2018, 49, 733–743. [Google Scholar]
- Anil, S.; Anand, P.; Alghamdi, H.; Jansen, J. Dental implant surface enhancement and osseointegration. In Implant Dentistry—A Rapidly Evolving Practice; Ilser, T., Ed.; IntechOpen: London, UK, 2011; pp. 83–108. [Google Scholar]
- Kieswetter, K.; Schwartz, Z.; Dean, D.; Boyan, B. The role of implant surface characteristics in the healing of bone. Crit. Rev. Oral Biol. Med. 1996, 7, 329–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traini, T.; Neugebauer, J.; Thams, U.; Zöller, J.E.; Caputi, S.; Piattelli, A. Peri-implant bone organization under immediate loading conditions: Collagen fiber orientation and mineral density analyses in the minipig model. Clin. Implant Dent. Relat. Res. 2009, 11, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ferguson, S.J.; Beutler, T.; Cochran, D.L.; Sittig, C.; Hirt, H.P.; Buser, D. Biomechanical comparison of the sandblasted and acid-etched and the machined and acid-etched titanium surface for dental implants. J. Biomed. Mater. Res. 2002, 60, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Piattelli, A.; Gehrke, P.; Carinci, F. Clinical outcome of 802 immediately loaded 2-stage submerged implants with a new grit-blasted and acid-etched surface: 12-month follow-up. Int. J. Oral Maxillofac. Implants 2006, 21, 763–768. [Google Scholar] [PubMed]
- Novaes, A.B., Jr.; Papalexiou, V.; Grisi, M.F.; Souza, S.S.; Taba, M., Jr.; Kajiwara, J.K. Influence of implant microstructure on the osseointegration of immediate implants placed in periodontally infected sites: A histomorphometric study in dogs. Clin. Oral Implants Res. 2004, 15, 34–43. [Google Scholar] [CrossRef]
- Traini, T.; Murmura, G.; Sinjari, B.; Perfetti, G.; Scarano, A.; D’Arcangelo, C.; Caputi, S. The surface anodization of titanium dental implants improves blood clot formation followed by osseointegration. Coatings 2018, 8, 252. [Google Scholar] [CrossRef] [Green Version]
- Sul, Y.-T.; Johansson, C.; Wennerberg, A.; Cho, L.-R.; Chang, B.-S.; Albrektsson, T. Optimum Surface Properties of Oxidized Implants for Reinforcement of Osseointeg ration: Surface Chemistry, Oxide Thickness, Porosity, Roughness, and Crystal Structure. Int. J. Oral Maxillofac. Implants 2005, 20, 349–359. [Google Scholar]
- Degidi, M.; Nardi, D.; Piattelli, A. 10-year follow-up of immediately loaded implants with TiUnite porous anodized surface. Clin. Implant Dent. Relat. Res. 2012, 14, 828–838. [Google Scholar] [CrossRef]
- Friberg, B.; Dahlin, C.; Widmark, G.; Östman, P.O.; Billström, C. One-Year Results of a Prospective Multicenter Study on Brånemark System® Implants with a TiUnite™ Surface. Clin. Implant Dent. Relat. Res. 2005, 7, s70–s75. [Google Scholar] [CrossRef]
- Jungner, M.; Lundqvist, P.; Lundgren, S. Oxidized titanium implants (Nobel Biocare® TiUnite™) compared with turned titanium implants (Nobel Biocare® mark III™) with respect to implant failure in a group of consecutive patients treated with early functional loading and two-stage protocol. Clin. Oral Implants Res. 2005, 16, 308–312. [Google Scholar] [CrossRef]
- Fröberg, K.K.; Lindh, C.; Ericsson, I. Immediate Loading of Brånemark System Implants®: A Comparison Between TiUniteTM and Turned Implants Placed in the Anterior Mandible. Clin. Implant Dent. Relat. Res. 2006, 8, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Sul, Y.-T.; Johansson, C.; Albrektsson, T. Which surface properties enhance bone response to implants? Comparison of oxidized magnesium, TiUnite, and Osseotite implant surfaces. Int. J. Prosthodont. 2006, 19, 319–329. [Google Scholar] [PubMed]
- Chou, W.-C.; Wang, R.C.-C.; Huang, C.-L.; Lee, T.-M. The effect of plasma treatment on the osseointegration of rough titanium implant: A histo-morphometric study in rabbits. J. Dent. Sci. 2018, 13, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Tallarico, M.; Gautier, G.; Mussano, F.; Botticelli, D. Plasma of argon affects the earliest biological response of different implant surfaces: An in vitro comparative study. J. Dent. Res. 2016, 95, 566–573. [Google Scholar] [CrossRef]
- Cochran, D.L. The scientific basis for and clinical experiences with Straumann implants including the ITI® Dental Implant System: A consensus report Note. Clin. Oral Implants Res. 2000, 11, 33–58. [Google Scholar] [CrossRef] [Green Version]
- Bernard, J.P.; Szmukler-Moncler, S.; Pessotto, S.; Vazquez, L.; Belser, U.C. The anchorage of Brånemark and ITI implants of different lengths. I. An experimental study in the canine mandible. Clin. Oral Implants Res. 2003, 14, 593–600. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Groetz, K.; Goetz, H.; Duschner, H.; Wagner, W. Comparative histomorphometry and resonance frequency analysis of implants with moderately rough surfaces in a loaded animal model. Clin. Oral Implants Res. 2008, 19, 1–8. [Google Scholar] [CrossRef]
- Mendonça, G.; Mendonça, D.B.; Aragao, F.J.; Cooper, L.F. Advancing dental implant surface technology–From micron-to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef]
- Monsees, T.K.; Barth, K.; Tippelt, S.; Heidel, K.; Gorbunov, A.; Pompe, W.; Funk, R.H. Effects of different titanium alloys and nanosize surface patterning on adhesion, differentiation, and orientation of osteoblast-like cells. Cells Tissues Organs 2005, 180, 81–95. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Vazquez, L.; Park, Y.-J.; Sammartino, G.; Bernard, J.-P. Identification card and codification of the chemical and morphological characteristics of 14 dental implant surfaces. J. Oral Implantol. 2011, 37, 525–542. [Google Scholar] [CrossRef]
- Jaeger, N.A.; Brunette, D.M. Production of microfabricated surfaces and their effects on cell behavior. In Titanium in Medicine; Brunette, D.M., Tengvall, P., Textor, M., Thomsen, P., Eds.; Springer-Verlag Berlin Heidelberg: Heidelberg, Germany, 2001; pp. 343–374. [Google Scholar]
- Nevins, M.; Nevins, M.L.; Camelo, M.; Boyesen, J.L.; Kim, D.M. Human histologic evidence of a connective tissue attachment to a dental implant. Int. J. Periodont. Restor. Dent. 2008, 28, 2. [Google Scholar]
- Pecora, G.E.; Ceccarelli, R.; Bonelli, M.; Alexander, H.; Ricci, J.L. Clinical evaluation of laser microtexturing for soft tissue and bone attachment to dental implants. Implant Dent. 2009, 18, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevins, M.; Kim, D.M.; Jun, S.-H.; Guze, K.; Schupbach, P.; Nevins, M.L. Histologic evidence of a connective tissue attachment to laser microgrooved abutments: A canine study. Int. J. Periodont. Restor. Dent. 2010, 30, 244–255. [Google Scholar]
- Farronato, D.; Mangano, F.; Briguglio, F.; Iorio-Siciliano, V.; Riccitiello, F.; Guarnieri, R. Influence of Laser-Lok surface on immediate functional loading of implants in single-tooth replacement: A 2-year prospective clinical study. Int. J. Periodont. Restor. Dent. 2014, 34, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-H.; Cho, S.-A. Comparison of removal torques for laser-treated titanium implants with anodized implants. J. Craniofac. Surg. 2011, 22, 1491–1495. [Google Scholar] [CrossRef]
- Rong, M.; Lu, H.; Wan, L.; Zhang, X.; Lin, X.; Li, S.; Zhou, L.; Lv, Y.; Su, Y. Comparison of early osseointegration between laser-treated/acid-etched and sandblasted/acid-etched titanium implant surfaces. J. Mater. Sci. Mater. Med. 2018, 29, 43. [Google Scholar] [CrossRef]
- Kopf, B.S.; Ruch, S.; Berner, S.; Spencer, N.D.; Maniura-Weber, K. The role of nanostructures and hydrophilicity in osseointegration: In-vitro protein-adsorption and blood-interaction studies. J. Biomed. Mater. Res. A 2015, 103, 2661–2672. [Google Scholar] [CrossRef]
- Kaluđerović, M.R.; Krajnović, T.; Maksimović-Ivanić, D.; Graf, H.-L.; Mijatović, S. Ti-SLActive and TiZr-SLActive Dental Implant Surfaces Promote Fast Osteoblast Differentiation. Coatings 2017, 7, 102. [Google Scholar] [CrossRef]
- Herrmann, J.; Hentschel, A.; Glauche, I.; Vollmer, A.; Schlegel, K.A.; Lutz, R. Implant survival and patient satisfaction of reduced diameter implants made from a titanium-zirconium alloy: A retrospective cohort study with 550 implants in 311 patients. J. Cranio-Maxillofac. Surg. 2016, 44, 1940–1944. [Google Scholar] [CrossRef]
- Rossi, F.; Lang, N.P.; Ricci, E.; Ferraioli, L.; Baldi, N.; Botticelli, D. Long-term follow-up of single crowns supported by short, moderately rough implants—A prospective 10-year cohort study. Clin. Oral Implants Res. 2018, 29, 1212–1219. [Google Scholar] [CrossRef]
- Schwarz, F.; Sager, M.; Ferrari, D.; Herten, M.; Wieland, M.; Becker, J. Bone regeneration in dehiscence-type defects at non-submerged and submerged chemically modified (SLActive®) and conventional SLA titanium implants: An immunohistochemical study in dogs. J. Clin. Periodont. 2008, 35, 64–75. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, H.; Raes, F.; Cooper, L.F.; Reside, G.; Garriga, J.S.; Tarrida, L.G.; Wiltfang, J.; Kern, M. Three-years clinical outcome of immediate provisionalization of single Osseospeed™ implants in extraction sockets and healed ridges. Clin. Oral Implants Res. 2013, 24, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, X.; Tang, Z.; Zhang, L.; Shi, D.; Meng, H. A prospective, multicenter study assessing the DENTSPLY Implants, OsseoSpeed™ TX, length 6 mm in the posterior maxilla and mandible: A 1-year follow-up study. Clin. Oral Implants Res. 2016, 27, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Rocci, M.; Rocci, A.; Martignoni, M.; Albrektsson, T.; Barlattani, A.; Gargari, M. Comparing the TiOblast and Osseospeed surfaces. Histomorphometric and histological analysis in humans. ORAL Implantol. 2008, 1, 34. [Google Scholar]
- Lee, S.Y.; Yang, D.J.; Yeo, S.; An, H.W.; Ryoo, K.H.; Park, K.B. The cytocompatibility and osseointegration of the Ti implants with XPEED ® surfaces. Clin. Oral Implants Res. 2012, 23, 1283–1289. [Google Scholar] [CrossRef]
- Barrere, F.; Van Der Valk, C.; Meijer, G.; Dalmeijer, R.; De Groot, K.; Layrolle, P. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats. J. Biomed. Mater. Res. B 2003, 67, 655–665. [Google Scholar] [CrossRef]
- Morris, H.F.; Ochi, S.; Spray, J.R.; Olson, J.W. Periodontal-Type Measurements Associated with Hydroxyapatite-Coated and Non—HA-Coated Implants: Uncovering to 36 Months. Ann. Periodontol. 2000, 5, 56–67. [Google Scholar] [CrossRef]
- Yu-Liang, C.; Lew, D.; Park, J.B.; Keller, J.C. Biomechanical and morphometric analysis of hydroxyapatite-coated implants with varying crystallinity. J. Oral Maxillofac. Surg. 1999, 57, 1096–1108. [Google Scholar] [CrossRef]
- Tinsley, D.; Watson, C.J.; Russell, J.L. A comparison of hydroxylapatite coated implant retained fixed and removable mandibular prostheses over 4 to 6 years. Clin. Oral Implants Res. 2001, 12, 159–166. [Google Scholar] [CrossRef]
- Van Oirschot, B.A.; Bronkhorst, E.M.; van den Beucken, J.J.; Meijer, G.; Jansen, J.; Junker, R. A systematic review on the long-term success of calcium phosphate plasma-spray-coated dental implants. Odontology 2016, 104, 347–356. [Google Scholar] [CrossRef]
- Herekar, M.G.; Patil, V.N.; Mulani, S.S.; Sethi, M.; Padhye, O. The influence of thread geometry on biomechanical load transfer to bone: A finite element analysis comparing two implant thread designs. Dent. Res. J. 2014, 11, 489. [Google Scholar]
- Cervino, G.; Romeo, U.; Lauritano, F.; Bramanti, E.; Fiorillo, L.; D’Amico, C.; Milone, D.; Laino, L.; Campolongo, F.; Rapisarda, S. Fem and von mises analysis of OSSTEM® dental implant structural components: Evaluation of different direction dynamic loads. Open Dent. J. 2018, 12, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.-H.; Kim, S.-Y.; Yi, Y.-J.; Lee, B.-K.; Kim, Y.-K. Hydroxyapatite-coated implant: Clinical prognosis assessment via a retrospective follow-up study for the average of 3 years. J. Adv. Prosthodont. 2018, 10, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-K.; Ahn, K.-J.; Yun, P.-Y.; Kim, M.; Yang, H.-S.; Yi, Y.-J.; Bae, J.-H. Effect of loading time on marginal bone loss around hydroxyapatite-coated implants. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Takayama, T.; Yoo, D.; Jimbo, R.; Karunagaran, S.; Tovar, N.; Janal, M.N.; Yamano, S. Nanometer-scale features on micrometer-scale surface texturing: A bone histological, gene expression, and nanomechanical study. Bone 2014, 65, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Bucci-Sabattini, V.; Cassinelli, C.; Coelho, P.G.; Minnici, A.; Trani, A.; Ehrenfest, D.M.D. Effect of titanium implant surface nanoroughness and calcium phosphate low impregnation on bone cell activity in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 217–224. [Google Scholar] [CrossRef]
- Mendes, V.C.; Moineddin, R.; Davies, J.E. The effect of discrete calcium phosphate nanocrystals on bone-bonding to titanium surfaces. Biomaterials 2007, 28, 4748–4755. [Google Scholar] [CrossRef]
- Goené, R.J.; Testori, T.; Trisi, P. Influence of a nanometer-scale surface enhancement on de novo bone formation on titanium implants: A histomorphometric study in human maxillae. Int. J. Periodont. Restor. Dent. 2007, 27, 3. [Google Scholar]
- Mendes, V.C.; Moineddin, R.; Davies, J.E. Discrete calcium phosphate nanocrystalline deposition enhances osteoconduction on titanium-based implant surfaces. J. Biomed. Mater. Res. Part. A 2009, 90, 577–585. [Google Scholar] [CrossRef]
- Telleman, G.; Meijer, H.; Vissink, A.; Raghoebar, G. Short implants with a nanometer-sized CaP surface provided with either a platform-switched or platform-matched abutment connection in the posterior region: A randomized clinical trial. Clin. Oral Implants Res. 2013, 24, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- Telleman, G.; Albrektsson, T.; Hoffman, M.; Johansson, C.B.; Vissink, A.; Meijer, H.J.; Raghoebar, G.M. Peri-implant endosseous healing properties of dual acid-etched mini-implants with a nanometer-sized deposition of CaP: A histological and histomorphometric human study. Clin. Implant Dent. Relat. Res. 2010, 12, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, E.A.; Granato, R.; Marin, C.; Jimbo, R.; Giro, G.; Suzuki, M.; Coelho, P.G. Biomechanical testing of microblasted, acid-etched/microblasted, anodized, and discrete crystalline deposition surfaces: An experimental study in beagle dogs. Int. J. Oral Maxillofac. Implants 2013, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, W.; Moore, L.V. The bacteria of periodontal diseases. Periodontology 1994, 5, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morse, D.J.; Wilson, M.J.; Wei, X.; Lewis, M.A.; Bradshaw, D.J.; Murdoch, C.; Williams, D.W. Denture-associated biofilm infection in three-dimensional oral mucosal tissue models. J. Med. Microbiol. 2018, 67, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements: A review. Acta Orthop. Scand. 2001, 72, 557–571. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef]
- Orapiriyakul, W.; Young, P.S.; Damiati, L.; Tsimbouri, P.M. Antibacterial surface modification of titanium implants in orthopaedics. J. Tissue Eng. 2018. [Google Scholar] [CrossRef] [Green Version]
- Kassem, A.; Lindholm, C.; Lerner, U.H. Toll-like receptor 2 stimulation of osteoblasts mediates Staphylococcus aureus induced bone resorption and osteoclastogenesis through enhanced RANKL. PLoS ONE 2016. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.J.; Ward, C.L.; Romano, D.R.; Hurtgen, B.J.; Hardy, S.K.; Woodbury, R.L.; Trevino, A.V.; Rathbone, C.R.; Wenke, J.C. Staphylococcus aureus biofilms decrease osteoblast viability, inhibits osteogenic differentiation, and increases bone resorption in vitro. BMC Musculoskelet. Disord. 2013, 14, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trouillet-Assant, S.; Gallet, M.; Nauroy, P.; Rasigade, J.-P.; Flammier, S.; Parroche, P.; Marvel, J.; Ferry, T.; Vandenesch, F.; Jurdic, P. Dual impact of live Staphylococcus aureus on the osteoclast lineage, leading to increased bone resorption. J. Infect. Dis. 2014, 211, 571–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duddeck, D.U.; Albrektsson, T.; Wennerberg, A.; Larsson, C.; Beuer, F. On the Cleanliness of Different Oral Implant Systems: A Pilot Study. J. Clin. Med. 2019, 8, 1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, T.G.; Valderrama, P.; Burbano, M.; Blansett, J.; Levine, R.; Kessler, H.; Rodrigues, D.C. Foreign bodies associated with peri-implantitis human biopsies. J. Periodontol. 2015, 86, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Korsch, M.; Obst, U.; Walther, W. Cement-associated peri-implantitis: A retrospective clinical observational study of fixed implant-supported restorations using a methacrylate cement. Clin. Oral Implants Res. 2014, 25, 797–802. [Google Scholar] [CrossRef]
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral biofilms: Development, control, and analysis. High-Throughput 2018, 7, 24. [Google Scholar]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S313–S318. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.; Lang, N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontology 2010, 53, 167–181. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alsabeeha, N.H.; Faggion, C.M.; Duncan, W.J. The frequency of peri-implant diseases: A systematic review and meta-analysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar] [CrossRef] [Green Version]
- Esposito, M.; Ardebili, Y.; Worthington, H.V. Interventions for replacing missing teeth: Different types of dental implants. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Doornewaard, R.; Christiaens, V.; De Bruyn, H.; Jacobsson, M.; Cosyn, J.; Vervaeke, S.; Jacquet, W. Long-term effect of surface roughness and patients’ factors on crestal bone loss at dental implants. A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2017, 19, 372–399. [Google Scholar] [CrossRef] [PubMed]
- Claffey, N.; Clarke, E.; Polyzois, I.; Renvert, S. Surgical treatment of peri-implantitis. J. Clin. Periodontol. 2008, 35, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Kotsovilis, S.; Karoussis, I.K.; Trianti, M.; Fourmousis, I. Therapy of peri-implantitis: A systematic review. J. Clin. Periodontol. 2008, 35, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implants 2010, 25, 63–74. [Google Scholar] [PubMed]
- Hickok, N.; Shapiro, I.; Chen, A. The impact of incorporating antimicrobials into implant surfaces. J. Dent. Res. 2018, 97, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Mas-Moruno, C.; Su, B.; Dalby, M.J. Multifunctional coatings and nanotopographies: Toward cell instructive and antibacterial implants. Adv. Healthc. Mater. 2019, 8, 1801103. [Google Scholar] [CrossRef]
- Alt, V. Antimicrobial coated implants in trauma and orthopaedics—A clinical review and risk-benefit analysis. Injury 2017, 48, 599–607. [Google Scholar] [CrossRef]
- Qin, H.; Cao, H.; Zhao, Y.; Jin, G.; Cheng, M.; Wang, J.; Jiang, Y.; An, Z.; Zhang, X.; Liu, X. Antimicrobial and osteogenic properties of silver-ion-implanted stainless steel. ACS Appl. Mater. Interfaces 2015, 7, 10785–10794. [Google Scholar] [CrossRef]
- Bai, L.; Hang, R.; Gao, A.; Zhang, X.; Huang, X.; Wang, Y.; Tang, B.; Zhao, L.; Chu, P.K. Nanostructured titanium–silver coatings with good antibacterial activity and cytocompatibility fabricated by one-step magnetron sputtering. Appl. Surf. Sci. 2015, 355, 32–44. [Google Scholar] [CrossRef]
- Qiao, S.; Cao, H.; Zhao, X.; Lo, H.; Zhuang, L.; Gu, Y.; Shi, J.; Liu, X.; Lai, H. Ag-plasma modification enhances bone apposition around titanium dental implants: An animal study in Labrador dogs. Int. J. Nanomed. 2015, 10, 653. [Google Scholar]
- Zhu, Y.; Cao, H.; Qiao, S.; Wang, M.; Gu, Y.; Luo, H.; Meng, F.; Liu, X.; Lai, H. Hierarchical micro/nanostructured titanium with balanced actions to bacterial and mammalian cells for dental implants. Int. J. Nanomed. 2015, 10, 6659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Dabbagh, A.; Razak, B.A.; Mahmoodian, R.; Nasiri-Tabrizi, B.; Hosseini, H.R.M.; Saber-Samandari, S.; Kasim, N.H.A.; Abdullah, H.; Sukiman, N.L. Highly-ordered TiO2 nanotubes decorated with Ag2O nanoparticles for improved biofunctionality of Ti6Al4V. Surf. Coat. Technol. 2018, 349, 1008–1017. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Huo, K.; Gao, B.; Xiong, W. Long-lasting in vivo and in vitro antibacterial ability of nanostructured titania coating incorporated with silver nanoparticles. J. Biomed. Mater. Res. A 2014, 102, 3488–3499. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, A.; Bai, L.; Wang, Y.; Wang, X.; Zhang, X.; Huang, X.; Hang, R.; Tang, B.; Chu, P.K. Antibacterial, osteogenic, and angiogenic activities of SrTiO3 nanotubes embedded with Ag2O nanoparticles. Mater. Sci. Eng. C 2017, 75, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xiong, W.; Fang, Z.; Guan, H.; Wu, W.; Li, Y.; Zhang, Y.; Alvarez, M.M.; Gao, B.; Huo, K. Strontium (Sr) and silver (Ag) loaded nanotubular structures with combined osteoinductive and antimicrobial activities. Acta Biomater. 2016, 31, 388–400. [Google Scholar] [CrossRef]
- Sarraf, M.; Dabbagh, A.; Razak, B.A.; Nasiri-Tabrizi, B.; Hosseini, H.R.M.; Saber-Samandari, S.; Kasim, N.H.A.; Yean, L.K.; Sukiman, N.L. Silver oxide nanoparticles-decorated tantala nanotubes for enhanced antibacterial activity and osseointegration of Ti6Al4V. Mater. Res. Des. 2018, 154, 28–40. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Ge, S.; Chen, J.; Ji, P. The relationship between substrate morphology and biological performances of nano-silver-loaded dopamine coatings on titanium surfaces. R. Soc. Open Sci. 2018, 5, 172310. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Shi, Y.; Cheng, Y.; Zheng, Y. Polydopamine-induced nanocomposite Ag/CaP coatings on the surface of titania nanotubes for antibacterial and osteointegration functions. J. Mater. Chem. B 2015, 3, 8796–8805. [Google Scholar] [CrossRef]
- Jin, G.; Cao, H.; Qiao, Y.; Meng, F.; Zhu, H.; Liu, X. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf. B Biointerfaces 2014, 117, 158–165. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, G.; Xue, Y.; Wang, D.; Liu, X.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Villa, D.; Jiménez Gómez-Lavín, M.; Abradelo, C.; San Román, J.; Rojo, L. Tissue engineering therapies based on folic acid and other vitamin B derivatives. Functional mechanisms and current applications in regenerative medicine. Int. J. Mol. Sci. 2018, 19, 4068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Tan, L.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Balancing bacteria–osteoblast competition through selective physical puncture and biofunctionalization of ZnO/polydopamine/arginine-glycine-aspartic acid-cysteine nanorods. ACS Nano 2017, 11, 11250–11263. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni Aranya, A.; Pushalkar, S.; Zhao, M.; LeGeros, R.Z.; Zhang, Y.; Saxena, D. Antibacterial and bioactive coatings on titanium implant surfaces. J. Biomed. Mater. Res. A 2017, 105, 2218–2227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Lu, Y.; Li, S.; Guo, S.; He, M.; Luo, K.; Lin, J. Copper-modified Ti6Al4V alloy fabricated by selective laser melting with pro-angiogenic and anti-inflammatory properties for potential guided bone regeneration applications. Mater. Sci. Eng. C 2018, 90, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, X.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Wu, H.; Feng, Q.; Liu, Y. The Cu-containing TiO2 coatings with modulatory effects on macrophage polarization and bactericidal capacity prepared by micro-arc oxidation on titanium substrates. Colloids Surf. B Biointerfaces 2018, 170, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, G.; Harja, M. Cerium-doped hydroxyapatite/collagen coatings on titanium for bone implants. Ceram. Int. 2019, 45, 2852–2857. [Google Scholar] [CrossRef]
- Moreira, H.; Costa-Barbosa, A.; Marques, S.M.; Sampaio, P.; Carvalho, S. Evaluation of cell activation promoted by tantalum and tantalum oxide coatings deposited by reactive DC magnetron sputtering. Surf. Coat. Technol. 2017, 330, 260–269. [Google Scholar] [CrossRef]
- Veerachamy, S.; Hameed, P.; Sen, D.; Dash, S.; Manivasagam, G. Studies on Mechanical, Biocompatibility and Antibacterial Activity of Plasma Sprayed Nano/Micron Ceramic Bilayered Coatings on Ti–6Al–4V Alloy for Biomedical Application. J. Nanosci. Nanotechnol. 2018, 18, 4515–4523. [Google Scholar] [CrossRef]
- Cheng, M.; Qiao, Y.; Wang, Q.; Qin, H.; Zhang, X.; Liu, X. Dual ions implantation of zirconium and nitrogen into magnesium alloys for enhanced corrosion resistance, antimicrobial activity and biocompatibility. Colloids Surf. B Biointerfaces 2016, 148, 200–210. [Google Scholar] [CrossRef]
- Bierbaum, S.; Mulansky, S.; Bognár, E.; Kientzl, I.; Nagy, P.; Vrana, N.E.; Weszl, M.; Boschke, E.; Scharnweber, D.; Wolf-Brandstetter, C. Osteogenic nanostructured titanium surfaces with antibacterial properties under conditions that mimic the dynamic situation in the oral cavity. Biomater. Sci. 2018, 6, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.; Abradelo, C.; San Román, J.; Rojo, L. Bibliographic review on the state of the art of strontium and zinc based regenerative therapies. Recent developments and clinical applications. J. Mater. Chem. B 2019, 7, 1974–1985. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Liu, S.; Wu, R.; Aparicio, C.; Wu, J. In vivo osseointegration of dental implants with an antimicrobial peptide coating. J. Mater. Sci. Mater. Med. 2017, 28, 76. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Junjian, C.; Chengzhi, C.; Lin, S.; Sa, L.; Li, R.; Yingjun, W. Multi-biofunctionalization of a titanium surface with a mixture of peptides to achieve excellent antimicrobial activity and biocompatibility. J. Mater. Chem. B 2015, 3, 30–33. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, F.J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial properties of hLf1–11 peptide onto titanium surfaces: A comparison study between silanization and surface initiated polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Godoy-Gallardo, M.; Guillem-Marti, J.; Sevilla, P.; Manero, J.M.; Gil, F.J.; Rodriguez, D. Anhydride-functional silane immobilized onto titanium surfaces induces osteoblast cell differentiation and reduces bacterial adhesion and biofilm formation. Mater. Sci. Eng. C 2016, 59, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Bronk, J.K.; Russell, B.H.; Rivera, J.J.; Pasqualini, R.; Arap, W.; Höök, M.; Barbu, E.M. A multifunctional streptococcal collagen-mimetic protein coating prevents bacterial adhesion and promotes osteoid formation on titanium. Acta Biomater. 2014, 10, 3354–3362. [Google Scholar] [CrossRef]
- Liu, H.-W.; Wei, D.-X.; Deng, J.-Z.; Zhu, J.-J.; Xu, K.; Hu, W.-H.; Xiao, S.-H.; Zhou, Y.-G. Combined antibacterial and osteogenic in situ effects of a bifunctional titanium alloy with nanoscale hydroxyapatite coating. Artif. Cells Nanomed. Biotechnol. 2018, 46, S460–S470. [Google Scholar] [CrossRef]
- Shahi, R.; Albuquerque, M.; Münchow, E.; Blanchard, S.; Gregory, R.; Bottino, M. Novel bioactive tetracycline-containing electrospun polymer fibers as a potential antibacterial dental implant coating. Odontology 2017, 105, 354–363. [Google Scholar] [CrossRef]
- Bottino, M.C.; Münchow, E.A.; Albuquerque, M.T.; Kamocki, K.; Shahi, R.; Gregory, R.L.; Chu, T.M.G.; Pankajakshan, D. Tetracycline-incorporated polymer nanofibers as a potential dental implant surface modifier. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2085–2092. [Google Scholar] [CrossRef]
- Gómez-Florit, M.; Monjo, M.; Ramis, J.M. Quercitrin for periodontal regeneration: Effects on human gingival fibroblasts and mesenchymal stem cells. Sci. Rep. 2015, 5, 16593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Córdoba, A.; Satué, M.; Gómez-Florit, M.; Hierro-Oliva, M.; Petzold, C.; Lyngstadaas, S.P.; González-Martín, M.L.; Monjo, M.; Ramis, J.M. Flavonoid-modified surfaces: Multifunctional bioactive biomaterials with osteopromotive, anti-inflammatory, and anti-fibrotic potential. Adv. Healthc. Mater. 2015, 4, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Somsanith, N.; Kim, Y.-K.; Jang, Y.-S.; Lee, Y.-H.; Yi, H.-K.; Jang, J.-H.; Kim, K.-A.; Bae, T.-S.; Lee, M.-H. Enhancing of Osseointegration with Propolis-Loaded TiO2 Nanotubes in Rat Mandible for Dental Implants. Mater. Des. 2018, 11, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Bano, S.; Ghosh, A.S.; Mandal, M.; Kim, H.-W.; Dey, T.; Kundu, S.C. Silk fibroin nanoparticles support in vitro sustained antibiotic release and osteogenesis on titanium surface. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1193–1204. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, Z.; Xiong, P.; Yan, J.; Li, Y.; Li, M.; Cheng, Y.; Zheng, Y. Bioinspired and biomimetic AgNPs/gentamicin-embedded silk fibroin coatings for robust antibacterial and osteogenetic applications. ACS Appl. Mater. Interfaces 2017, 9, 25830–25846. [Google Scholar] [CrossRef]
- De Cremer, K.; Braem, A.; Gerits, E.; De Brucker, K.; Vandamme, K.; Martens, J.; Michiels, J.; Vleugels, J.; Cammue, B.; Thevissen, K. Controlled release of chlorhexidine from a mesoporous silica-containing macroporous titanium dental implant prevents microbial biofilm formation. Eur. Cells Mater. 2017, 33, 13–27. [Google Scholar] [CrossRef]
- Ao, H.; Zhou, J.; Tang, T.; Yue, B. Biofunctionalization of titanium with bacitracin immobilization shows potential for anti-bacteria, osteogenesis and reduction of macrophage inflammation. Colloids Surf. B Biointerfaces 2016, 145, 728–739. [Google Scholar]
- Wei, Q.; Becherer, T.; Noeske, P.L.M.; Grunwald, I.; Haag, R. A universal approach to crosslinked hierarchical polymer multilayers as stable and highly effective antifouling coatings. Adv. Mater. 2014, 26, 2688–2693. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, F.; Li, K.; Qin, C.; Ma, P.; Dai, L.; Cai, K. Cecropin B loaded TiO2 nanotubes coated with hyaluronidase sensitive multilayers for reducing bacterial adhesion. Mater. Des. 2016, 92, 1007–1017. [Google Scholar] [CrossRef]
- Haas, S.; Hain, N.; Raoufi, M.; Handschuh-Wang, S.; Wang, T.; Jiang, X.; Schönherr, H. Enzyme degradable polymersomes from hyaluronic acid-block-poly (ε-caprolactone) copolymers for the detection of enzymes of pathogenic bacteria. Biomacromolecules 2015, 16, 832–841. [Google Scholar] [CrossRef]
- Baier, G.; Cavallaro, A.; Vasilev, K.; Mailänder, V.; Musyanovych, A.; Landfester, K. Enzyme responsive hyaluronic acid nanocapsules containing polyhexanide and their exposure to bacteria to prevent infection. Biomacromolecules 2013, 14, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Huang, S.; Lan, S.; Xiong, H.; Tao, B.; Ding, Y.; Liu, Y.; Liu, P.; Cai, K. Surface engineering of titanium implants with enzyme-triggered antibacterial properties and enhanced osseointegration in vivo. J. Mater. Chem. B 2018, 6, 8090–8104. [Google Scholar] [CrossRef]
- Rojo, L.; Gharibi, B.; McLister, R.; Meenan, B.J.; Deb, S. Self-assembled monolayers of alendronate on Ti6Al4V alloy surfaces enhance osteogenesis in mesenchymal stem cells. Sci. Rep. 2016, 6, 30548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo, L.; Deb, S. Polymer therapeutics in relation to dentistry. In Biomaterials for Oral and Craniomaxillofacial Applications; Deb, S., Ed.; Karger Publishers: Basel, Switzerland, 2015; Volume 17, pp. 13–21. [Google Scholar]
- Yang, M.; Jiang, P.; Ge, Y.; Lan, F.; Zhou, X.; He, J.; Wu, Y. Dopamine self-polymerized along with hydroxyapatite onto the preactivated titanium percutaneous implants surface to promote human gingival fibroblast behavior and antimicrobial activity for biological sealing. J. Biomater. Appl. 2018, 32, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Neoh, K.; Kang, E.; Poh, C.K.; Wang, W. Surface functionalization of titanium with carboxymethyl chitosan and immobilized bone morphogenetic protein-2 for enhanced osseointegration. Biomacromolecules 2009, 10, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Neoh, K.G.; Kang, E.-T. Bifunctional coating based on carboxymethyl chitosan with stable conjugated alkaline phosphatase for inhibiting bacterial adhesion and promoting osteogenic differentiation on titanium. Appl. Surf. Sci. 2016, 360, 86–97. [Google Scholar] [CrossRef]
- Palla-Rubio, B.; Araújo-Gomes, N.; Fernández-Gutiérrez, M.; Rojo, L.; Suay, J.; Gurruchaga, M.; Goñi, I. Synthesis and characterization of silica-chitosan hybrid materials as antibacterial coatings for titanium implants. Carbohydr. Polym. 2019, 203, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, H.; Ghasemi, Z.; Kharaziha, M.; Karimzadeh, F.; Alihosseini, F. Chitosan-58S bioactive glass nanocomposite coatings on TiO2 nanotube: Structural and biological properties. Appl. Surf. Sci. 2018, 441, 138–149. [Google Scholar] [CrossRef]











| Manufacturing Technique | Example of Commercial Brand |
|---|---|
| Sandblasting | TiOblast® (Astra Tech, Mölndal, Sweden), Swede and Screw Vent® (Zimmer Biomet, Palm Beach Gardens, Florida, USA) and Standard, Hex® (Osteoplant, Poznan, Poland). |
| Acid-etching | Osseotite® (Zimmer Biomet, Warsaw, Indiana, USA) and Steri-Oss Etched® (Nobel Biocare, Zürich-Flughafen, Switzerland) |
| Grit blasting and acid-etching | SLA Straumann® (Straumann Institute, Basel, Switzerland), Ankylos® (Dentsply Friadent, Mannheim, Germany), Friadent Plus® (Dentsply Friadent, Mannheim, Germany), Promote® (Camlog, Basel, Switzerland) and Osseonova® (Ziacom, Pinto, Spain) |
| Anodization | TiUnite® (Nobel Biocare, Gothenburg, Sweden) |
| Plasma spraying | IMZ-TPS® (Dentsply Friadent, Mannhein, Germany), Bonefit® (Straumann Institute, Waldenburg, Switzerland), Restore-TPS® (Lifecore Biomedical, Chaska, Minnesota, USA), Steri-Oss-TPS® (Nobel Biocare, Yorba Linda, California, USA) and ITI-TPS® (Straumann Institute, Waldenburg, Germany) |
| Manufacturing Technique | Examples of Commercial Brand |
|---|---|
| Laser Ablation | Laser-Lok® (BioHorizons, Birmingham, Alabama) |
| Grit blasting and acid etching | SLActive® (Straumann Institute, Basel, Switzerland), Osseospeed® (Dentsply Friadent, Mannheim, Germany) |
| Coating Component | Examples of Commercial Brand |
|---|---|
| Hap | Osstem TS III-HA® (Osstem Implant Co., Busan, Korea) and Osstem GS-HA III® (Osstem Implant Co., Busan, Korea), TSV-HA® (Zimmer Biomet, Carlsbad, California, USA) |
| CaP nanoparticles | Ossean® (Intra-Lock, Boca Raton, Florida, USA) |
| CaP and DCD nanotopography | Nanotite® (Zimmer Biomet, Palm Beach Gardens, Florida, USA) |
| Nanostructured Ca | XPEED® (MegaGen Implant Co., Gyeongbuk, South Korea) |
| Surface Modification Process | ||||||
|---|---|---|---|---|---|---|
| Commercial Brand | Sandblasting | Acid Etching | Anodization | Plasma Spraying | CDC | |
| Smooth Surface (SA = 0–0.5 µm) | Brånemark® | |||||
| Minimally Rough (SA = 0.5–1 µm) | Osseotite® | × | ||||
| Nanotite® | × | × | ||||
| Moderately Rough (SA = 1–2 µm) | TiOblast® | × | ||||
| Steri-Oss Etched® | × | |||||
| SLA Straumann® | × | × | ||||
| SLActive® | × | × | ||||
| Osseospeed® | × | × | ||||
| Rough Surfaces (SA > 2 µm) | Ankylos® | × | × | |||
| Friadent Plus® | × | × | ||||
| TiUnite® | × | |||||
| ITI-TPS® | × | |||||
| TSV-HA® | × | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asensio, G.; Vázquez-Lasa, B.; Rojo, L. Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces. J. Clin. Med. 2019, 8, 1982. https://doi.org/10.3390/jcm8111982
Asensio G, Vázquez-Lasa B, Rojo L. Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces. Journal of Clinical Medicine. 2019; 8(11):1982. https://doi.org/10.3390/jcm8111982
Chicago/Turabian StyleAsensio, Gerardo, Blanca Vázquez-Lasa, and Luis Rojo. 2019. "Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces" Journal of Clinical Medicine 8, no. 11: 1982. https://doi.org/10.3390/jcm8111982
APA StyleAsensio, G., Vázquez-Lasa, B., & Rojo, L. (2019). Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces. Journal of Clinical Medicine, 8(11), 1982. https://doi.org/10.3390/jcm8111982