Circulating miRNAs as Biomarkers for Endometriosis and Endometriosis-Related Ovarian Cancer—An Overview
Abstract
:1. Introduction
2. Material and Methods
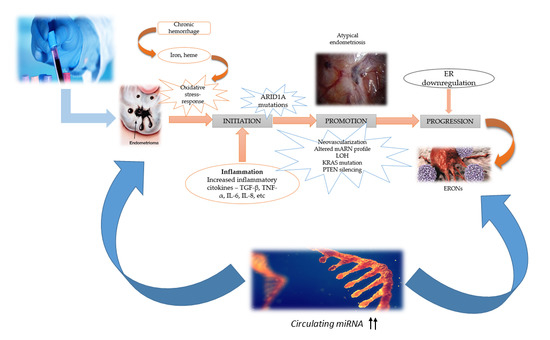
3. Molecular Mechanism and Pathogenesis of ERONs Development
4. ARID1A Mutations in ERONs
5. PIK3/AKT-Pathway Mutations in ERONs
6. Circulating miRNAs as Biomarkers in Endometriosis
6.1. miRNAs—An Overview of Biogenesis and Function
6.2. miRNA Sequencing Methods
7. Results and Discussion
7.1. Differential miRNAs Expression in Endometriosis
7.2. miRNAs Dysregulations in Endometriosis
7.3. Circulating miRNAs as Biomarkers in ERONs and Ovarian Cancer
8. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| ARID1A | AT-rich interactive domain-containing protein 1A |
| BRAF | V-raf murine sarcoma viral oncogene homolog B1 |
| CA-125 | Cancer-associated carbohydrate antigen 125 |
| CCC | Clear cell carcinoma |
| CLIC4 | Chloride intracellular channel 4 |
| CTNNB1 | Gene encoding catenin beta-1 |
| DGCR8 | Double stranded RNA binding protein |
| DNA | Deoxyribonucleic acid |
| EAOC | Endometriosis-associated ovarian carcinoma |
| EnOC | Endometrioid ovarian carcinoma |
| EOC | Epithelial ovarian cancer |
| ER | Estrogen-receptors |
| ERONs | Endometriosis-related ovarian neoplasms |
| Exp-5 | Exportin 5 |
| FGF-2 | Fibroblast growth factor-2 |
| HDL | High density lipoprotein |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| KRAS | V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog |
| let-7 | A micro-RNA precursor |
| miR | Micro- RNA precursor family |
| miRNA | Micro Ribonucleic acid |
| MMPs | Matri metalloproteinases |
| p53 | Protein 53 |
| PI3K | Phosphatidylinositol 3-kinase |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| Pol II | RNA Polymerase II |
| PPP2R1A | Protein phosphatase two scaffold subunit Aalpha |
| PTEN | Phosphatase and tensin homolog |
| qRT-PCR | Quantitative Reverse transcription polymerase chain reaction |
| RCBTB1 | Regulator of chromosome condensation and BTB domain-containing protein-1 |
| RISC | RNA induced silencing complex |
| rRNA | Ribosomal RNA |
| RT-PCR | Reverse transcription polymerase chain reaction |
| shRNA | Short hairpin RNA |
| siRNA | Short interfering RNA |
| SMAD3 | Mothers against decapentaplegic homolog 3 |
| snRNA | Small nuclear RNA |
| snoRNA | Small nucleolar RNA |
| SMARCB1 | A gene that encodes SWI/SNF related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 |
| stRNA | Small temporal RNA |
| SWI/SNF | Switch/Sucrose Non-Fermentable |
| TGF-β | Transforming growth factor beta |
| TNF-α | Tumor necrosis factor alpha |
| TP53 | Tumor protein p53 |
| tRNA | Transfer RNA |
| WWOX | Ww domain-containing oxidoreductase |
References
- Johnson, N.P.; Hummelshoj, L. Consensus on current management of endometriosis. Hum. Reprod. 2013, 28, 1552–1568. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Singh, V.; Tayade, C. Biomarkers in endometriosis: Challenges and opportunities. Fertil. Steril. 2017, 107, 523–532. [Google Scholar] [CrossRef]
- Farland, L.V.; Horne, A.W. Disparity in endometriosis diagnoses between racial/ethnic groups. BJOG Int. J. Obstet. Gynaecol. 2019. Peer-review version. [Google Scholar] [CrossRef] [PubMed]
- Cramer, D.W.; Missmer, S.A. The epidemiology of endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Fuldeore, M.J.; Soliman, A.M. Prevalence and Symptomatic Burden of Diagnosed Endometriosis in the United States: National Estimates from a Cross-Sectional Survey of 59,411 Women. Gynecol. Obstet. Investig. 2017, 82, 453–461. [Google Scholar] [CrossRef]
- Sayasneh, A.; Tsivos, D.; Crawford, R. Endometriosis and ovarian cancer: A systematic review. ISRN Obstet. Gynecol. 2011, 2011, 140310. [Google Scholar] [CrossRef]
- Lapp, T. ACOG issues recommendations for the management of endometriosis. American College of Obstetricians and Gynecologists. Am. Fam. Phys. 2000, 62, 1431–1434. [Google Scholar]
- Saridogan, E. Adolescent endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Karnezis, A.N.; Cho, K.R.; Gilks, C.B.; Pearce, C.L.; Huntsman, D.G. The disparate origins of ovarian cancers: Pathogenesis and prevention strategies. Nat. Rev. Cancer 2017, 17, 65–74. [Google Scholar] [CrossRef]
- Thomas, E.J.; Campbell, I.G. Evidence that endometriosis behaves in a malignant manner. Gynecol. Obstet. Investig. 2000, 50, 2–10. [Google Scholar] [CrossRef]
- Sampson, J. Endometrial carcinoma of the ovary arising in endometrial tissue of that organ. Arch. Surg. 1925, 10, 1–72. [Google Scholar] [CrossRef]
- Swiersz, L.M. Role of Endometriosis in Cancer and Tumor Development. Ann. N. Y. Acad. Sci. 2002, 955, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Králíčková, M.; Vetvicka, V. Endometriosis and ovarian cancer. World J. Clin. Oncol. 2014, 10, 800–805. [Google Scholar] [CrossRef]
- Scott, R.B. Malignant changes in endometriosis. Obstet. Gynecol. 1953, 2, 283–289. [Google Scholar]
- Maiorana, A.; Cicerone, C.; Niceta, M.; Alio, L. Evaluation of serum CA 125 levels in patients with pelvic pain related to endometriosis. Int. J. Biol. Mark. 2007, 22, 200–202. [Google Scholar] [CrossRef]
- Modesitt, S.C.; Tortolero-Luna, G.; Robinson, J.B.; Gershenson, D.M.; Wolf, J.K. Ovarian and extraovarian endometriosisassociated cancer. Obstet. Gynecol. 2002, 100, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Van Gorp, T.; Amant, F.; Neven, P.V. Endometriosis and the development of malignant tumors of the pelvis. A review of literature. Best Pract. Res. Clin. Obstet. Gynecol. 2004, 18, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.M. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef]
- Daichi, M.; Le-Ming, S. Pathogenesis and the role of ARID1A mutation in endometriosisrelated ovarian neoplasms. Adv. Anat. Pathol. 2013, 20, 45–52. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Arvatescu, C.A.; Ifteni, P.; Ples, L. Anticancer activity of toxins from bee and snake venom—an overview on ovarian cancer. Molecules 2018, 23, 692. [Google Scholar] [CrossRef]
- Birrer, M.J. The origin of ovarian cancer—Is it getting clearer? N. Engl. J. Med. 2010, 363, 1574–1575. [Google Scholar] [CrossRef] [PubMed]
- Nissenblatt, M. Endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 2011, 364, 482–483. [Google Scholar] [CrossRef]
- Kobayashi, H. Ovarian cancer in endometriosis: Epidemiology, natural history, and clinical diagnosis. Int. J. Clin. Oncol. 2009, 14, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, F.; Datta, S.; Hanson, V.; Pejovic, T.; Nezhat, C.; Nezhat, C. The relationship of endometriosis and ovarian malignancy: A review. Fertil. Steril. 2008, 90, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Moga, M.; Ples, L.; Bigiu, N.; Manitiu, I. An overview of the risk of adverse reproductive and developmental disorders due to exposure to pesticides. J. Environ. Prot. Ecol. 2011, 12, 1311–1319. [Google Scholar]
- Tanase, Y.; Yamada, Y.; Shigetomi, H.; Kajihara, H.; Oonogi, A.; Yoshizawa, Y.; Furukawa, N.; Haruta, S.; Yoshida, S.; Sado, T.; et al. Modulation of estrogenic action in clear cell carcinoma of the ovary (Review). Exp. Ther. Med. 2012, 3, 18–24. [Google Scholar] [CrossRef]
- Nakagawa, K.; Hisano, M.; Sugiyama, R.; Yamaguchi, K. Measurement of oxidative stress in the follicular fluid of infertility patients with an endometrioma. Arch. Gynecol. Obstet. 2016, 293, 197–202. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kajiwara, H.; Kanayama, S.; Yamada, Y.; Furukawa, N.; Noguchi, T. Molecular pathogenesis of endometriosis-associated clear cell carcinoma of the ovary (Review). Oncol. Rep. 2009, 22, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.K.L.; Wei, N.; Liu, S.S.; Xiao-Yun, L.M.P.; Cheung, A.N.; Ngan, H.Y.S. Estrogen Receptor Subtypes in Ovarian Cancer: A Clinical Correlation. Obstet. Gynecol. 2008, 111, 144–151. [Google Scholar] [CrossRef]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Wicklund, K.G.; Hein, R.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef]
- Sieh, W.; Kobel, M.; Longacre, T.A.; Bowtell, D.D.; Defazio, A.; Goodman, M.T.; Hogdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B.; et al. Hormone-receptor expression and ovarian cancer survival: An ovarian tumor tissue analysis consortium study. Lancet Oncol. 2013, 14, 853–862. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer-shifting the paradigm. Hum. Pathol. 2011, 42, 918–931. [Google Scholar] [CrossRef]
- Wei, J.J.; William, J.; Bulun, S. Endometriosis and Ovarian Cancer: A Review of Clinical, Pathologic, and Molecular Aspects. Int. J. Gynecol. Pathol. 2011, 30, 553–568. [Google Scholar] [CrossRef]
- Samartzis, E.P.; Noske, A.; Dedes, K.J.; Fink, D.; Imesch, P. ARID1A Mutations and PI3K/AKT Pathway Alterations in Endometriosis and Endometriosis-Associated Ovarian Carcinomas. Int. J. Mol. Sci. 2013, 14, 18824–18849. [Google Scholar] [CrossRef] [Green Version]
- Bukhari, S.A.; Ali, M.; Anwar, H.; Farooq, M.; Ercisli, S.; Dima, L.; Zia-Ul-Haq, M. Antioxidant potential of Cichorium intybus and Lentinus edodes ameloriates carbontetrachloride-induced liver toxicity. Oxid. Commun. 2015, 38, 2006–2015. [Google Scholar]
- Barreta, A.; Sarian, L.O.; Ferracini, A.C.; Costa, L.B.E.; Mazzola, P.G.; de Angelo, A.L.; Derchain, S. Immunohistochemistry expression of targeted therapies biomarkers in ovarian clear cell and endometriod carcinomas (type I) and endometriois. Hum. Pathol. 2019, 85, 72–81. [Google Scholar] [CrossRef]
- Cornen, S.; Adelaide, J.; Bertucci, F.; Finetti, P.; Guille, A.; Birnbaum, D.J.; Birnbaum, D.; Chaffanet, M. Mutations and deletions of arid1a in breast tumors. Oncogene 2012, 31, 4255–4256. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M.; Ushigome, S. Epithelial metaplastic changes in ovarian endometriosis. Mod. Pathol. 1998, 11, 784–788. [Google Scholar]
- Wu, J.N.; Roberts, C.W. Arid1a mutations in cancer: Another epigenetic tumor suppressor? Cancer Discov. 2013, 3, 35–43. [Google Scholar] [CrossRef]
- Rutgers, J.L.; Scully, R.E. Ovarian mullerian mucinous papillary cystadenomas of borderline malignancy. A clinicopathologic analysis. Cancer 1988, 61, 340–348. [Google Scholar] [CrossRef]
- Tan, D.S.; Kaye, S. Ovarian clear cell adenocarcinoma: A continuing enigma. J. Clin. Pathol. 2007, 60, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Suryo Rahmanto, Y.; Wu, R.C.; Wang, Y.; Wang, Z.; Wang, T.L.; Shih, I.M. Roles of deletion of Arid1a, a tumor suppressor, in mouse ovarian tumorigenesis. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Wang, T.L.; Shih, M. ARID1A, a Factor That Promotes Formation of SWI/SNF-Mediated Chromatin Remodeling, Is a Tumor Suppressor in Gynecologic Cancers. Cancer Mol. 2011, 71, 6718–6727. [Google Scholar] [CrossRef]
- Bosse, T.; Ter Haar, N.T.; Seeber, L.M.; Diest, P.J.; Hes, F.J.; Vasen, H.F.; Nout, R.A.; Creutzberg, C.L.; Morreau, H.; Smit, V.T. Loss of arid1a expression and its relationship with pi3k-akt pathway alterations, tp53 and microsatellite instability in endometrial cancer. Mod. Pathol. 2013, 26, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Feroze-Zaidi, F.; Fusi, L.; Takano, M.; Higham, J.; Salker, M.S.; Goto, T.; Edassery, S.; Klingel, K.; Boini, K.M.; Palmada, M.; et al. Role and regulation of the serum- and glucocorticoid-regulated kinase 1 in fertile and infertile human endometrium. Endocrinology 2007, 148, 5020–5029. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.T.; Mao, T.L.; Jones, S.; Veras, E.; Ayhan, A.; Wang, T.L.; Glas, R.; Slamon, D.; Velculescu, V.E.; Kuman, R.J.; et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am. J. Pathol. 2009, 174, 1597–1601. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Hull, M.L. Tissue and circulating microRNA influence reproductive function in endometrial tissue. Reprod. BioMed. Online 2013, 27, 515–529. [Google Scholar] [CrossRef]
- Hammond, S.M. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Condorelli, G.; Dimmeler, S. MicroRNAs: Components of an integrated system controlling cardiac development, physiology, and disease pathogenesis. Cardiovasc. Res. 2008, 79, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, K.; Sivasankar, V. MicroRNAs-Biology and clinical applications. J. Oral Maxillofac. Pathol. 2014, 18, 229–234. [Google Scholar] [CrossRef]
- Saini, H.K.; Griffiths-Jones, S.; Enright, A.J. Genomic analysis of human microRNA transcripts. Proc. Natl. Acad. Sci. USA 2007, 104, 17719–17724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattick, J.S.; Makunin, I.V. Small regulatory RNAs in mammals. Hum. Mol. Genet. 2005, 14, 121–132. [Google Scholar] [CrossRef]
- Shukla, G.C.; Singh, J.; Barik, S. MicroRNAs: Processing, maturation, target recognition and regulatory functions. Mol. Cell. Pharmacol. 2011, 3, 83–92. [Google Scholar] [CrossRef]
- Hammond, S.M. RNAi, microRNAs, and human disease. Cancer Chemother. Pharmacol. 2006, 58, S63–S68. [Google Scholar] [CrossRef]
- Bhayani, M.K.; Calin, G.A.; Lai, S.Y. Functional relevance of miRNA sequences in human disease. Mutat. Res. 2012, 731, 14–19. [Google Scholar] [CrossRef]
- Agrawal, S.; Tapmeier, T.T.; Rahmioglu, N.; Kirtley, S.; Zondervan, K.T.; Becker, C.M. The miRNA Mirage: How Close Are We to Finding a Non-Invasive Diagnostic Biomarker in Endometriosis? A Systematic Review. Int. J. Mol. Sci. 2018, 19, 599. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, B.; Jeang, K.T. RISCy business: MicroRNAs, pathogenesis, and viruses. J. Biol. Chem. 2007, 282, 26641–26645. [Google Scholar] [CrossRef] [PubMed]
- Turchinovich, A.; Weiz, L.; Langheinz, A.; Burwinkel, B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011, 39, 7223–7233. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef]
- Lee, Y.; Jeon, K.; Lee, J.T.; Kim, S.; Kim, V.N. MicroRNA maturation: Stepwise processing and subcellular localization. EMBO J. 2002, 21, 4663–4670. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lee, Y.; Yeom, K.H.; Kim, Y.K.; Jin, H.; Kim, V.N. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004, 18, 3016–3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landthaler, M.; Yalcin, A.; Tuschl, T. The human DiGeorge syndrome critical region gene 8 and its D. melanogaster homolog are required for miRNA biogenesis. Curr. Biol. 2004, 14, 2162–2167. [Google Scholar] [CrossRef]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Kim, S.; Kim, V.N.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hagedorn, C.H.; Cullen, B.R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 2004, 10, 1957–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, E.; Guttinger, S.; Calado, A.; Dahlberg, J.E.; Kutay, U. Nuclear export of microRNA precursors. Science 2004, 303, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Fortin, K.; Mourelatos, Z. MicroRNAs: Biogenesis and Molecular Functions. Brain Pathol. 2008, 18, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.I.; Chendrimada, T.P.; Cooch, N.; Shiekhattar, R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 2005, 123, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Rajewsky, N.; Socci, N.D. Computational identification of microRNA targets. Dev. Biol. 2004, 267, 529–535. [Google Scholar] [CrossRef]
- Lewis, B.P.; Shih, I.H.; Jones-Rhoades, M.W.; Bartel, D.P.; Burge, C.B. Prediction of mammalian microRNA targets. Cell 2003, 115, 787–798. [Google Scholar] [CrossRef]
- Ferlita, A.; Battaglia, R.; Andronico, F.; Caruso, S.; Cianci, A.; Purello, M.; Pietro, C.D. Non-Coding RNAs in Endometrial Physiopathology. Int. J. Mol. Sci. 2018, 19, 2120. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, L.; Batte, K.E.; Trgovcich, J.; Wisler, J.; Marsh, C.B.; Piper, M. Methodological challenges in utilizing miRNAs as circulating biomarkers. J. Cell. Mol. Med. 2014, 18, 371–390. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.A.; Calin, G.A. MicroRNA identification in plasma and serum: A new tool to diagnose and monitor diseases. Expert Opin. Biol. Ther. 2009, 9, 703–711. [Google Scholar] [CrossRef]
- D’haene, B.; Vandesompele, J.; Hellemans, J. Accurate and objective copy number profiling using real-time quantitative PCR. Methods 2010, 50, 262–270. [Google Scholar] [CrossRef]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem–loop RT–PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Lee, E.J.; Jiang, J.; Sarkar, A.; Yang, L.; Elton, T.S.; Chen, C. Real-time PCR quantification of precursor and mature microRNA. Methods 2008, 44, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Ji, T.; Zheng, Z.G.; Wang, F.M.; Xu, L.J.; Li, L.F.; Cheng, Q.H.; Guo, J.F.; Ding, X.F. Differential microRNA expression by Solexa sequencing in the sera of ovarian cancer patients. APJCP 2014, 15, 1739–1743. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA profiling: Approaches and considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef]
- Guo, Z.; Maki, M.; Ding, R.; Yang, Y.; Zhang, B.; Xiong, L. Genome-wide survey of tissue-specific microRNA and transcription factor regulatory networks in 12 tissues. Sci. Rep. 2014, 4, 5150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. ThemicroRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Ohlsson Teague, E.M.C.; Print, C.G.; Hull, M.L. The role of microRNAs in endometriosis and associated reproductive conditions. Hum. Reprod. Update 2009, 16, 142–165. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, S.M.; Creighton, C.J.; Matzuk, M.M. Functional MicroRNA Involved in Endometriosis Results Clinical demographics Endometriomas have dysregulated microRNA expression. Mol. Cell. Endocrinol. 2011, 5, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum. Reprod. 2012, 27, 712–721. [Google Scholar] [CrossRef]
- Jia, S.Z.; Yang, Y.; Lang, J.; Sun, P.; Leng, J. Plasma miR-17–5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Hum. Reprod. 2013, 28, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, W.; Ren, C.; Zhao, M.; Jiang, X.; Fang, X.; Xia, X. Analysis of serum microRNA profile by Solexa sequencing in women with endometriosis. Reprod. Sci. 2016. [Google Scholar] [CrossRef]
- Zhao, M.; Tang, Q.; Wu, W.; Xia, Y.; Chen, D.; Wang, X. miR-20a contributes to endometriosis by regulating NTN4 expression. Mol. Biol. Rep. 2014, 41, 5793–5797. [Google Scholar] [CrossRef]
- Zheng, B.; Xue, X.; Zhao, Y.; Chen, J.; Xu, C.-Y.; Duan, P. The differential expression of microRNA-143,145 in endometriosis. Iran. J. Reprod. Med. 2014, 12, 555–560. [Google Scholar] [PubMed]
- Wang, W.T.; Zhao, Y.N.; Han, B.W.; Hong, S.J.; Chen, Y.Q. Circulating MicroRNAs Identified in a Genome-Wide Serum MicroRNA Expression Analysis as Noninvasive Biomarkers for Endometriosis. J. Clin. Endocrinol. Metab. 2013, 98, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.Y.; Hsieh, T.H.; Tsai, C.F.; Tsai, H.P.; Chen, H.S.; Chang, Y.; Chuang, H.Y.; Lee, J.N.; Hsu, Y.L.; Tsai, E.M. MiRNA-199a-5p regulates VEGFA in endometrial mesenchymal stem cells and contributes to the pathogenesis of endometriosis. J. Pathol. 2014, 232, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gu, L.; Di, W. MiR-199a attenuates endometrial stromal cell invasiveness through suppression of the IKKβ/nf-κb pathway and reduced interleukin-8 expression. Mol. Hum. Reprod. 2012, 18, 136–145. [Google Scholar] [CrossRef]
- Cosar, E.; Mamillapalli, R.; Ersoy, G.S.; Cho, S.; Seifer, B.; Taylor, H.S. Serum microRNAs as diagnostic markers of endometriosis: A comprehensive array-based analysis. Fertil. Steril. 2016, 106, 402–409. [Google Scholar] [CrossRef]
- Cho, S.; Mutlu, L.; Grechukhina, O.; Taylor, H. Circulating microRNAs as potential biomarkers for endometriosis. Fertil. Steril. 2015, 103, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, S.; Vlad, A.M.; Lin, H.M.; Mantia-Smaldone, G.; Laskey, R.; Lee, M.; Lin, Y.; Donnellan, N.; Klein-Patel, M.; Lee, T.; et al. Plasma MicroRNAs as Novel Biomarkers for Endometriosis and Endometriosis-Associated Ovarian Cancer. Clin. Cancer Res. 2013, 19, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Rekker, K.; Saare, M.; Roost, A.M.; Kaart, T.; Sõritsa, D.; Karro, H.; Sõritsa, A.; Simón, C.; Salumets, A.; Peters, M. Circulating miR-200-family micro-RNAs have altered plasma levels in patients with endometriosis and vary with blood collection time. Fertil. Steril. 2015, 104, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Sharkey, D.J.; Wang, Z.; Evans, S.F.; Healey, M.; Ohlsson, E.M.C.; Print, C.G.; Robertson, S.A.; Hull, M.L. Article Navigation. Plasma miRNAs Display Limited Potential as Diagnostic Tools for Endometriosis. J. Clin. Endocrinol. Metab. 2019, 104, 1999–2022. [Google Scholar] [CrossRef] [PubMed]
- Marí-Alexandre, J.; García-Oms, J.; Barceló-Molina, M.; Gilabert-Aguilar, J.; Estellés, A.; Braza-Boïls, A.; Gilabert-Estellés, J. MicroRNAs and angiogenesis in endometriosis. Thromb. Res. 2015, 135, S38–S40. [Google Scholar] [CrossRef]
- Josep, M.A.; Sanchez-Izquierdo, D.; Estelles, J.; Barcelo-Molina, M.; Braza-Boils, A.; Sandoval, J. miRNAs Regulation and Its Role as Biomarkers in Endometriosis. Int. J. Mol. Sci. 2016, 17, 93. [Google Scholar] [CrossRef]
- Silva, S.S.; Lopes, C.; Teixeira, A.L.; Carneiro de Sousa, M.J.; Medeiros, R. Forensic miRNA: Potential biomarker for body fluids? Forensic Sci. Int. Genet. 2015, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Seifer, B.J.; Su, D.; Taylor, H.S. Circulating miRNAs in Murine Experimental Endometriosis: Decreased Abundance of let-7a. Reprod. Sci. 2017, 24, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Laudanski, P.; Charkiewicz, R.; Kuzmicki, M.; Szamatowicz, J.; Charkiewicz, A.; Niklinski, J. MicroRNAs expression profiling of eutopic proliferative endometrium in women with ovarian endometriosis. Reprod. Biol. Endocrinol. 2013, 11, 78. [Google Scholar] [CrossRef]
- Grechukhina, O.; Petracco, R.; Popkhadze, S.; Massasa, E.; Paranjape, T.; Chan, E.; Flores, I.; Weidhaas, J.B.; Taylor, H.S. A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis. EMBO Mol. Med. 2012, 4, 206–217. [Google Scholar] [CrossRef]
- Cosar, E.; Mamillapalli, R.; Moridi, I.; Duleba, A.; Taylor, H.S. Serum MicroRNA Biomarkers Regulated by Simvastatin in a Primate Model of Endometriosis. Reprod. Sci. 2018, 1933719118765971. [Google Scholar] [CrossRef]
- Burney, R.O.; Hamilton, A.E.; Aghajanova, L.; Vo, K.C.; Nezhat, C.N.; Lessey, B.A.; Giudice, L.C. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol. Hum. Reprod. 2009, 15, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Gilabert-Estelles, J.; Braza-Boils, A.; Ramon, L.A.; Zorio, E.; Medina, P.; Espana, F.; Estelles, A. Role of microRNAs in gynecological pathology. Curr. Med. Chem. 2012, 19, 2406–2413. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [Green Version]
- Häusler, S.F.M.; Keller, A.; Chandran, P.A.; Ziegler, K.; Zipp, K.; Heuer, S.; Krockenberger, M.; Engel, J.B.; Hönig, A.; Scheffler, M. Whole blood-derived miRNA profiles as potential new tools for ovarian cancer screening. Br. J. Cancer 2010, 103, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Kinose, Y.; Sawada, K.; Nakamura, K.; Kimura, T. The role of microRNAs in ovarian cancer. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, L.; Zhao, Y.; Yang, D.; Song, F.; Wen, Y.; Hao, Q.; Hu, Z.; Zhang, W.; Chen, K. Plasma miRNAs as diagnostic and prognostic biomarkers for ovarian cancer. PLoS ONE 2013, 8, e77853. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.W.; Hahn, M.A.; Gard, G.B.; Maidens, J.; Huh, J.Y.; Marsh, D.J.; Howell, V.M. Elevated levels of circulating microRNA-200 family members correlate with serous epithelial ovarian cancer. BMC Cancer 2012, 12, 627. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Tian, J.; Lin, Y.; Jin, Y.; Wang, L.; Cui, M. Serum microRNA-92 expression in patients with ovarian epithelial carcinoma. J. Int. Med. Res. 2013, 41, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Lai, C.H.; Chen, H.C.; Lin, C.Y.; Tsai, C.L.; Tang, Y.H.; Huang, H.J.; Lin, C.T.; Chen, M.Y.; Huang, K.G.; et al. Serum microRNAs in clear cell carcinoma of the ovary. Taiwan. J. Obstet. Gynecol. 2014, 53, 536–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resnick, K.E.; Alder, H.; Hagan, J.P.; Richardson, D.L.; Croce, C.M.; Cohn, D.E. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol. Oncol. 2009, 112, 55–59. [Google Scholar] [CrossRef]
- Shapira, I.; Oswald, M.; Lovecchio, J.; Khalili, H.; Menzin, A.; Whyte, J.; Dos Santos, L.; Liang, S.; Bhuiya, T.; Keogh, M.; et al. Circulating biomarkers for detection of ovarian cancer and predicting cancer outcomes. Br. J. Cancer 2014, 110, 976–983. [Google Scholar] [CrossRef] [PubMed]

| Author, Reference | miRNA | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Jia, 2013 [88] | miR-20a | 60 | 90 |
| miR-22 | 90 | 90 | |
| miR-17-5p | 60 | 80 | |
| Cosar, 2016 [95] | mi-125b-5p | 100 | 96 |
| Cho, 2015 [96] | let-7d | 83.3 | 100 |
| Wang, 2013 [92] | miR-122 | 80 | 76 |
| miR-141-5p | 71.69 | 96 | |
| miR-145 | 70 | 96 | |
| miR-199a | 78.33 | 76 | |
| Suryavanshi, 2013 [97] | miR-16+miR-191+miR-195 | 88 | 60 |
| Rekker, 2015 [98] | miR-141 | 71.9 | 70.8 |
| miR-200a | 90.6 | 62.5 | |
| miR-200b | 90.6 | 70.8 | |
| Nisenblat, 2019 [99] | miR-155+miR574-3p+miR139-3p | 83 | 51 |
| Author, Year, Reference | Biofluid | Cases | mRNAs Sequencing Method | Results |
|---|---|---|---|---|
| Wang, 2016 [89] | Serum | 30 cases of stage I-II endometriosis and 20 controls | Deep sequencing | Up-regulated: miR-185-5p, miR-242-5p, miR-296-5p, miR-3127-5p, miR-424-3p, miR-4645-3p, miR-502-3p, miR-542-3p, miR-550a-3p, miR-636 |
| Hsu, 2014 [93] | Serum | 40 cases of endometriosis and 25 controls | Array profiling | Down-regulated: mir-199a-5p |
| Cosar, 2016 [95] | Serum | 24 cases of stage III-IV endometriosis and 24 controls | Array profiling | Down-regulated: miR-3613-5p, miR-6755-3p Up-regulated: miR-500a-3p, miR-451a, miR-18a-5p, miR-342-3p, miR-125b-5p. |
| Burney, 2009 [107] | Serum | 4 cases of endometriosis and 3 controls | Array profiling | Down-regulated: miR-34c-3p, miR-9*, miR-34b*, miR-34c-5p, miR-9 |
| Suryawanshi, 2013 [97] | Plasma | 33 cases of and 20 controls | Array profiling | Up-regulated: miR-16, miR-191, miR-195 |
| Cho, 2015 [96] | Serum | 24 cases of stage III-IV endometriosis and 24 controls | Targeted | Down-regulated: let7b, miR-125a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moga, M.A.; Bălan, A.; Dimienescu, O.G.; Burtea, V.; Dragomir, R.M.; Anastasiu, C.V. Circulating miRNAs as Biomarkers for Endometriosis and Endometriosis-Related Ovarian Cancer—An Overview. J. Clin. Med. 2019, 8, 735. https://doi.org/10.3390/jcm8050735
Moga MA, Bălan A, Dimienescu OG, Burtea V, Dragomir RM, Anastasiu CV. Circulating miRNAs as Biomarkers for Endometriosis and Endometriosis-Related Ovarian Cancer—An Overview. Journal of Clinical Medicine. 2019; 8(5):735. https://doi.org/10.3390/jcm8050735
Chicago/Turabian StyleMoga, Marius Alexandru, Andreea Bălan, Oana Gabriela Dimienescu, Victoria Burtea, Roxana Maria Dragomir, and Costin Vlad Anastasiu. 2019. "Circulating miRNAs as Biomarkers for Endometriosis and Endometriosis-Related Ovarian Cancer—An Overview" Journal of Clinical Medicine 8, no. 5: 735. https://doi.org/10.3390/jcm8050735
APA StyleMoga, M. A., Bălan, A., Dimienescu, O. G., Burtea, V., Dragomir, R. M., & Anastasiu, C. V. (2019). Circulating miRNAs as Biomarkers for Endometriosis and Endometriosis-Related Ovarian Cancer—An Overview. Journal of Clinical Medicine, 8(5), 735. https://doi.org/10.3390/jcm8050735