Methylene Blue—Current Knowledge, Fluorescent Properties, and Its Future Use
Abstract
:1. Introduction
2. Fluorescence
3. Fluorescent Clinical Use
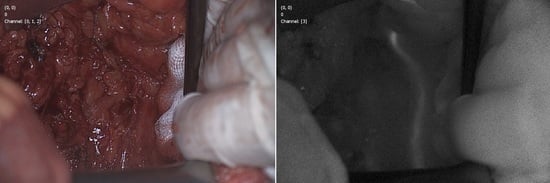
3.1. Visualization of Ureters
3.2. Thyroid and Parathyroid Glands
3.3. Pancreatic Neuroendocrine Tumor
3.4. Breast
4. Side Effects
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alkan Gürsel, S.; Yang, Z.; Choudhury, B.; Roelofs, M.G.; Scherer, G.G. Radiation-Grafted Membranes Using a Trifluorostyrene Derivative. J. Electrochem. Soc. 2006. [Google Scholar] [CrossRef]
- Salah, M.; Samy, N.; Fadel, M. Methylene blue mediated photodynamic therapy for resistant plaque psoriasis. J. Drugs Dermatol. 2009, 81, 42. [Google Scholar]
- ASTM International. Standard Test Method for Rapid Determination of the Methylene Blue Value for Fine Aggregate or Mineral Filler Using a Colorimeter; ASTM C1777-15; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Brayfield, A. Methylthioninium chloride. In Martindale: The Complete Drug Reference; Pharmaceutical Press: London, UK, 1883. [Google Scholar]
- Mathelin, C.; Croce, S.; Brasse, D.; Gairard, B.; Gharbi, M.; Andriamisandratsoa, N.; Bekaert, V.; Francis, Z.; Guyonnet, J.L.; Huss, D.; et al. Methylene blue dye, an accurate dye for sentinel lymph node identification in early breast cancer. Anticancer Res. 2009, 29, 4119–4125. [Google Scholar] [PubMed]
- Chen, W.; Chen, L.; Yang, S.; Chen, Z.; Qian, G.; Zhang, S.; Jing, J. A novel technique for localization of small pulmonary nodules. Chest 2007. [Google Scholar] [CrossRef] [PubMed]
- Prokop, E.K.; Buddemeyer, E.U.; Strauss, H.W.; Wagner, H.N. Detection and localization of an occult vesicoenteric fistula. Am. J. Roentgenol. 1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja, S.; Rice, T.W.; Neumann, D.R.; Saha, G.B.; Khandekar, S.; MacIntyre, W.J.; Go, R.T. Scintigraphic detection of post-pneumonectomy bronchopleural fistulae. Eur. J. Nucl. Med. 1999. [Google Scholar] [CrossRef]
- Chang, T.W.; Weinstein, L. Eczema herpeticum. Treatment with methylene blue and light. Arch. Dermatol. 1975, 111, 1174–1175. [Google Scholar] [CrossRef] [PubMed]
- Aghahosseini, F.; Arbabi-Kalati, F.; Fashtami, L.A.; Fateh, M.; Djavid, G.E. Treatment of oral lichen planus with photodynamic therapy mediated methylene blue: A case report. Med. Oral Patol. Oral Cir. Bucal. 2006, 11, E126–E129. [Google Scholar]
- Biel, M.A. Photodynamic therapy of bacterial and fungal biofilm infections. Methods Mol. Biol. 2010, 635, 175–194. [Google Scholar] [CrossRef]
- Lyon, J.P.; De Pedroso e Silva Azevedo, C.M.; Moreira, L.M.; De Lima, C.J.; De Resende, M.A. Photodynamic antifungal therapy against chromoblastomycosis. Mycopathologia 2011, 172, 293–297. [Google Scholar] [CrossRef]
- Marotti, J.; Sperandio, F.F.; Fregnani, E.R.; Aranha, A.C.C.; de Freitas, P.M.; de Paula Eduardo, C. High-intensity laser and photodynamic therapy as a treatment for recurrent herpes labialis. Photomed. Laser Surg. 2010, 28, 439–444. [Google Scholar] [CrossRef]
- Shannon, M.W.; Borron, S.W.B.M. Haddad and Winchester’s Clinical Management of Poisoning and Drug Overdose, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Bradberry, S.M. Occupational methaemoglobinaemia. Mechanisms of production, features, diagnosis and management including the use of methylene blue. Toxicol. Rev. 2003, 22, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Hamel, J. A review of acute cyanide poisoning with a treatment update. Crit. Care Nurse 2011, 31, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Peter, C.; Hongwan, D.; Küpfer, A.; Lauterburg, B.H. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur. J. Clin. Pharmacol. 2000. [Google Scholar] [CrossRef]
- Bai, S.W.; Huh, E.H.; Park, J.H.; Rha, K.H.; Kim, S.K.; Park, K.H. Urinary tract injuries during pelvic surgery: Incidence rates and predisposing factors. Int. Urogynecol. J. 2006. [Google Scholar] [CrossRef]
- Oz, M.; Lorke, D.E.; Hasan, M.; Petroianu, G.A. Cellular and molecular actions of Methylene Blue in the nervous system. Med. Res. Rev. 2011, 31, 93–117. [Google Scholar] [CrossRef] [Green Version]
- Akorn Inc. Methylene Blue Injection, USP 1% Prescribing Information; Akorn Inc.: Lake Forest, IL, USA, 2011. [Google Scholar]
- Jangjoo, A.; Forghani, M.N.; Mehrabibahar, M.; Sadeghi, R. Anaphylaxis reaction of a breast cancer patient to methylene blue during breast surgery with sentinel node mapping. Acta Oncol. 2010. [Google Scholar] [CrossRef]
- Gould, E.A.; Winship, T.; Philbin, P.H.; Kerr, H.H. Observations on a “sentinel node” in cancer of the parotid. Cancer 1960. [Google Scholar] [CrossRef]
- Cabanas, R.M. An approach for the treatment of penile carcinoma. Cancer 1977. [Google Scholar] [CrossRef]
- Chung, A.; Giuliano, A.E. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. In The Breast: Comprehensive Management of Benign and Malignant Diseases; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Morton, D.L.; Wen, D.R.; Wong, J.H.; Economou, J.S.; Cagle, L.A.; Storm, F.K.; Foshag, L.J.; Cochran, A.J. Technical Details of Intraoperative Lymphatic Mapping for Early Stage Melanoma. Arch. Surg. 1992. [Google Scholar] [CrossRef]
- Hillary, S.L.; Guillermet, S.; Brown, N.J.; Balasubramanian, S.P. Use of methylene blue and near-infrared fluorescence in thyroid and parathyroid surgery. Langenbeck’s Arch. Surg. 2018, 403, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulsow, J.; Winter, D.C.; O’Keane, J.C.; O’Connell, P.R. Sentinel lymph node mapping in colorectal cancer. Br. J. Surg. 2003, 90, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Kirgan, D.M.; Guenther, J.M.; Morton, D.L. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann. Surg. 1994, 220, 391–401. [Google Scholar] [CrossRef]
- Nedu, M.-E.; Tertis, M.; Cristea, C.; Georgescu, A.V. Comparative Study Regarding the Properties of Methylene Blue and Proflavine and Their Optimal Concentrations for In Vitro and In Vivo Applications. Diagnostics 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.Q.M.; McWade, M.; Thomas, G.; Beddard, B.; Herington, J.L.; Paria, B.C.; Schwartz, H.S.; Halpern, J.L.; Holt, G.E.; Mahadevan-Jansen, A. Development of a modular fluorescence overlay tissue imaging system for wide-field intraoperative surgical guidance. J. Med. Imaging 2018. [Google Scholar] [CrossRef]
- Vahrmeijer, A.L.; Hutteman, M.; Van der Vorst, J.R.; Van de Velde, C.J.H.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013. [Google Scholar] [CrossRef] [Green Version]
- Van Manen, L.; Handgraaf, H.J.M.; Diana, M.; Dijkstra, J.; Ishizawa, T.; Vahrmeijer, A.L.; Mieog, J.S.D. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J. Surg. Oncol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Schaafsma, B.E.; Mieog, J.S.D.; Hutteman, M.; Van der Vorst, J.R.; Kuppen, P.J.; Löwik, C.W.; Frangioni, J.V.; Van de Velde, C.J.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Polom, K.; Murawa, D.; Nowaczyk, P.; Rho, Y.S.; Murawa, P. Breast cancer sentinel lymph node mapping using near infrared guided indocyanine green and indocyanine green-human serum albumin in comparison with gamma emitting radioactive colloid tracer. Eur. J. Surg. Oncol. 2012. [Google Scholar] [CrossRef]
- Ginimuge, P.R.; Jyothi, S.D. Methylene blue: Revisited. J. Anaesthesiol. Clin. Pharmacol. 2010, 26, 517–520. [Google Scholar]
- Barnes, T.G.; Hompes, R.; Birks, J.; Mortensen, N.J.; Jones, O.; Lindsey, I.; Guy, R.; George, B.; Cunningham, C.; Yeung, T.M. Methylene blue fluorescence of the ureter during colorectal surgery. Surg. Endosc. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvam, S.; Sarkar, I. Bile salt induced solubilization of methylene blue: Study on methylene blue fluorescence properties and molecular mechanics calculation. J. Pharm. Anal. 2017, 7, 71–75. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006. [Google Scholar] [CrossRef]
- Shimizu, S.; Kamiike, W.; Hatanaka, N.; Yoshida, Y.; Tagawa, K.; Miyata, M.; Matsuda, H. New method for measuring ICG Rmax with a clearance meter. World J. Surg. 1995. [Google Scholar] [CrossRef]
- Al-Awadi, K.A.; Kehinde, E.O.; Al-Hunayan, A.; Al-Khayat, A. Iatrogenic ureteric injuries: Incidence, aetiological factors and the effect of early management on subsequent outcome. Int. Urol. Nephrol. 2005. [Google Scholar] [CrossRef]
- Palaniappa, N.C.; Telem, D.A.; Ranasinghe, N.E.; Divino, C.M. Incidence of iatrogenic ureteral injury after laparoscopic colectomy. Arch. Surg. 2012. [Google Scholar] [CrossRef]
- Mahendran, H.A.; Singam, P.; Ho, C.; Hong, G.E.; Hee, T.G.; Md Zainuddin, Z. Iatrogenic ureter injuries: Eleven years experience in a tertiary hospital. Med. J. Malays. 2012, 67, 169. [Google Scholar]
- Marcelissen, T.A.T.; Den Hollander, P.P.; Tuytten, T.R.A.H.; Sosef, M.N. Incidence of Iatrogenic Ureteral Injury during Open and Laparoscopic Colorectal Surgery: A Single Center Experience and Review of the Literature. Surg. Laparosc. Endosc. Percutaneous Tech. 2016. [Google Scholar] [CrossRef]
- Heald Mchir, R.J. The “Holy Plane” of Rectal Surgery. J. R. Soc. Med. 1988, 81, 503–508. [Google Scholar] [CrossRef]
- Delacroix, S.E.; Winters, J.C. Urinary tract injures: Recognition and management. Clin. Colon Rectal Surg. 2010. [Google Scholar] [CrossRef] [Green Version]
- Chahin, F.; Dwivedi, A.J.; Paramesh, A.; Chau, W.; Agrawal, S.; Chahin, C.; Kumar, A.; Tootla, A.; Tootla, F.; Silva, Y.J. The implications of lighted ureteral stenting in laparoscopic colectomy. J. Soc. Laparoendosc. Surg. 2002, 6, 49. [Google Scholar]
- Verbeek, F.P.R.; Van der Vorst, J.R.; Schaafsma, B.E.; Swijnenburg, R.J.; Gaarenstroom, K.N.; Elzevier, H.W.; van de Velde, C.J.; Frangioni, J.V.; Vahrmeijer, A.L. Intraoperative near infrared fluorescence guided identification of the ureters using low dose methylene blue: A first in human experience. J. Urol. 2013. [Google Scholar] [CrossRef] [Green Version]
- Al-Taher, M.; van den Bos, J.; Schols, R.M.; Bouvy, N.D.; Stassen, L.P.S. Fluorescence Ureteral Visualization in Human Laparoscopic Colorectal Surgery Using Methylene Blue. J. Laparoendosc. Adv. Surg. Tech. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.M.; Volpi, D.; Tullis, I.D.C.; Nicholson, G.A.; Buchs, N.; Cunningham, C.; Guy, R.; Lindsey, I.; George, B.; Jones, O.; et al. Identifying ureters in situ under fluorescence during laparoscopic and open colorectal surgery. Ann. Surg. 2016. [Google Scholar] [CrossRef] [PubMed]
- Matsui, A.; Tanaka, E.; Choi, H.S.; Kianzad, V.; Gioux, S.; Lomnes, S.J.; Frangioni, J.V. Real-time, near-infrared, fluorescence-guided identification of the ureters using methylene blue. Surgery 2010. [Google Scholar] [CrossRef] [Green Version]
- Bach, K.K.; Lindsay, F.W.; Berg, L.S.; Howard, R.S. Prolonged postoperative disorientation after methylene blue infusion during parathyroidectomy. Anesth. Analg. 2004. [Google Scholar] [CrossRef]
- Majithia, A.; Stearns, M.P. Methylene blue toxicity following infusion to localize parathyroid adenoma. J. Laryngol. Otol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; North, A.P.; Chadwick, D.R. Prolonged postoperative altered mental status after methylene blue infusion during parathyroidectomy: A case report and review of the literature. Ann. R. Coll. Surg. Engl. 2007. [Google Scholar] [CrossRef]
- Slooter, M.D.; Janssen, A.; Bemelman, W.A.; Tanis, P.J.; Hompes, R. Currently available and experimental dyes for intraoperative near-infrared fluorescence imaging of the ureters: A systematic review. Tech. Coloproctol. 2019. [Google Scholar] [CrossRef] [Green Version]
- McWade, M.A.; Thomas, G.; Nguyen, J.Q.; Sanders, M.E.; Solórzano, C.C.; Mahadevan-Jansen, A. Enhancing Parathyroid Gland Visualization Using a Near Infrared Fluorescence-Based Overlay Imaging System. J. Am. Coll. Surg. 2019. [Google Scholar] [CrossRef]
- Dudley, N.E. Methylene Blue for Rapid Identification of the Parathyroids. Br. Med. J. 1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, B.J.; Triponez, F. Intraoperative adjuncts in surgery for primary hyperparathyroidism. Langenbeck’s Arch. Surg 2009. [Google Scholar] [CrossRef]
- De Leeuw, F.; Breuskin, I.; Abbaci, M.; Casiraghi, O.; Mirghani, H.; Lakhdar, A.B.; Laplace-Builhé, C.; Hartl, D. Intraoperative Near-infrared Imaging for Parathyroid Gland Identification by Auto-fluorescence: A Feasibility Study. World J. Surg. 2016. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, D.; Huang, B.; Wang, C.; Zhao, L.; Xie, X.; Zhang, Z.; Wang, K.; Tian, J.; Luo, Y. Methylene Blue-Based Near-Infrared Fluorescence Imaging for Breast Cancer Visualization in Resected Human Tissues. Technol. Cancer Res. Treat. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, T.; Wang, X.; Li, J.; Zhao, H. Real-time near-infrared fluorescence imaging mediated by blue dye in breast cancer patients. J. Surg. Oncol. 2020, 121, 964–966. [Google Scholar] [CrossRef]
- Hariharan, U.; Sood, R.; Choudhury, A.; Garg, R.; Kaur, J. Oxygen desaturation following methylene blue injection: Not always spurious. Saudi J. Anaesth. 2011. [Google Scholar] [CrossRef]
- Varon, A.J.; Anderson, H.B.; Civetta, J.M. Desaturation noted by pulmonary artery catheter oximeter after methylene blue injection. Anesthesiology 1989. [Google Scholar] [CrossRef]
- Salhab, M.; Al Sarakbi, W.; Mokbel, K. Skin and fat necrosis of the breast following methylene blue dye injection for sentinel node biopsy in a patient with breast cancer. Int. Semin. Surg. Oncol. 2005. [Google Scholar] [CrossRef] [Green Version]
- Perry, P.; Meinhard, E. Necrotic subcutaneous abscesses following injections of methylene blue. Br. J. Clin. Pract. 1974, 28, 289. [Google Scholar]
- Varghese, P.; Abdel-Rahman, A.T.; Akberali, S.; Mostafa, A.; Gattuso, J.M.; Carpenter, R. Methylene blue dye—A safe and effective alternative for sentinel lymph node localization. Breast J. 2008, 14, 61–67. [Google Scholar] [CrossRef]
- Patel, H.P.; Chadwick, D.R.; Harrison, B.J.; Balasubramanian, S.P. Systematic review of intravenous methylene blue in parathyroid surgery. Br. J. Surg. 2012. [Google Scholar] [CrossRef]
- Vutskits, L.; Briner, A.; Klauser, P.; Gascon, E.; Dayer, A.G.; Kiss, J.Z.; Muller, D.; Licker, M.J.; Morel, D.R. Adverse effects of methylene blue on the central nervous system. Anesthesiology 2008. [Google Scholar] [CrossRef] [Green Version]
- Sidi, A.; Paulus, D.A.; Rush, W.; Gravenstein, N.; Davis, R.F. Methylene Blue and Indocyanine Green Artfactually Lower Pulse Oximetry Readings of Oxygen Saturation. Studies in Dogs. J. Clin. Monit. 1987. [Google Scholar] [CrossRef]
- Paras, C.; Keller, M.; White, L.; Phay, J.; Mahadevan-Jansen, A. Near-infrared autofluorescence for the detection of parathyroid glands. J. Biomed. Opt. 2011. [Google Scholar] [CrossRef] [PubMed]
- McWade, M.A.; Paras, C.; White, L.M.; Phay, J.E.; Mahadevan-Jansen, A.; Broome, J.T. A novel optical approach to intraoperative detection of parathyroid glands. Surgery 2013. [Google Scholar] [CrossRef] [Green Version]
- Van der Vorst, J.R.; Schaafsma, B.E.; Verbeek, F.P.R.; Swijnenburg, R.J.; Tummers, Q.R.; Hutteman, M.; Hamming, J.F.; Kievit, J.; Frangioni, J.V.; van de Velde, C.J.; et al. Intraoperative near-infrared fluorescence imaging of parathyroid adenomas with use of low-dose methylene blue. Head Neck 2014. [Google Scholar] [CrossRef] [Green Version]
- Winer, J.H.; Choi, H.S.; Gibbs-Strauss, S.L.; Ashitate, Y.; Colson, Y.L.; Frangioni, J.V. Intraoperative localization of insulinoma and normal pancreas using invisible near-infrared fluorescent light. Ann. Surg. Oncol. 2010. [Google Scholar] [CrossRef]
- Gordon, D.L.; Airan, M.C.; Suvanich, S. Visual identification of an insulinoma using methylene blue. Br. J. Surg. 1974. [Google Scholar] [CrossRef]
- Handgraaf, H.J.M.; Boogerd, L.S.F.; Shahbazi Feshtali, S.; Sarasqueta, A.F.; Snel, M.; Swijnenburg, R.J.; Vahrmeijer, A.L.; Bonsing, B.A.; Mieog, J.S.D. Intraoperative Near-Infrared Fluorescence Imaging of Multiple Pancreatic Neuroendocrine Tumors: A Case Report. Pancreas 2018. [Google Scholar] [CrossRef]
- Van der Vorst, J.R. Near-infrared fluorescence imaging of a solitary fibrous tumor of the pancreas using methylene blue. World J. Gastrointest. Surg. 2012. [Google Scholar] [CrossRef]
- Tummers, Q.R.J.G.; Boonstra, M.C.; Frangioni, J.V.; van de Velde, C.J.H.; Vahrmeijer, A.L.; Bonsing, B.A. Intraoperative near-infrared fluorescence imaging of a paraganglioma using methylene blue: A case report. Int. J. Surg. Case Rep. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwer de Koning, S.G.; Vrancken Peeters, M.J.T.F.D.; Jóźwiak, K.; Bhairosing, P.A.; Ruers, T.J.M. Tumor Resection Margin Definitions in Breast-Conserving Surgery: Systematic Review and Meta-analysis of the Current Literature. Clin. Breast Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Houvenaeghel, G.; Lambaudie, E.; Bannier, M.; Rua, S.; Barrou, J.; Heinemann, M.; Buttarelli, M.; Piana, J.T.; Cohen, M. Positive or close margins: Reoperation rate and second conservative resection or total mastectomy? Cancer Manag. Res. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tummers, Q.R.J.G.; Verbeek, F.P.R.; Schaafsma, B.E.; Boonstra, M.C.; van der Vorst, J.R.; Liefers, G.J.; van de Velde, C.J.; Frangioni, J.V.; Vahrmeijer, A.L. Real-time intraoperative detection of breast cancer using near-infrared fluorescence imaging and Methylene Blue. Eur. J. Surg. Oncol. 2014, 40, 850–858. [Google Scholar] [CrossRef] [Green Version]
- Bézu, C.; Coutant, C.; Salengro, A.; Daraï, E.; Rouzier, R.; Uzan, S. Anaphylactic response to blue dye during sentinel lymph node biopsy. Surg. Oncol. 2011. [Google Scholar] [CrossRef]
- Muñiz-Diaz, E.; Puig, L. Allergic and anaphylactic reactions to methylene-blue-treated plasma in Catalonia in the period 2008–2013. Blood Transfus. 2014. [Google Scholar] [CrossRef]
- Barras, A.C.H.; Walder, B.; Seeck, M. Serotonin syndrome following methylene blue infusion: A rare complication of antidepressant therapy. J. Neurol. Neurosurg. Psychiatry 2010. [Google Scholar] [CrossRef]
- Polymedica Pharmaceuticals USA Inc. Product Information. Urised Tablets (Methylene Blue); Polymedica Pharmaceuticals USA Inc.: Woburn, MA, USA, 1990. [Google Scholar]
- Youngster, I.; Arcavi, L.; Schechmaster, R.; Akayzen, Y.; Popliski, H.; Shimonov, J.; Beig, S.; Berkovitch, M. Medications and glucose-6-phosphate dehydrogenase deficiency: An evidence-based review. Drug Saf. 2010. [Google Scholar] [CrossRef]
- Porat, R.; Gilbert, S.; Magilner, D. Methylene blue-induced phototoxicity: An unrecognized complication. Pediatrics 1996, 97, 717–721. [Google Scholar]
- George, M. Methylene-blue-induced hyperbilirubinemia and phototoxicity in a neonate. Clin. Pediatr. 2000. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cwalinski, T.; Polom, W.; Marano, L.; Roviello, G.; D’Angelo, A.; Cwalina, N.; Matuszewski, M.; Roviello, F.; Jaskiewicz, J.; Polom, K. Methylene Blue—Current Knowledge, Fluorescent Properties, and Its Future Use. J. Clin. Med. 2020, 9, 3538. https://doi.org/10.3390/jcm9113538
Cwalinski T, Polom W, Marano L, Roviello G, D’Angelo A, Cwalina N, Matuszewski M, Roviello F, Jaskiewicz J, Polom K. Methylene Blue—Current Knowledge, Fluorescent Properties, and Its Future Use. Journal of Clinical Medicine. 2020; 9(11):3538. https://doi.org/10.3390/jcm9113538
Chicago/Turabian StyleCwalinski, Tomasz, Wojciech Polom, Luigi Marano, Giandomenico Roviello, Alberto D’Angelo, Natalia Cwalina, Marcin Matuszewski, Franco Roviello, Janusz Jaskiewicz, and Karol Polom. 2020. "Methylene Blue—Current Knowledge, Fluorescent Properties, and Its Future Use" Journal of Clinical Medicine 9, no. 11: 3538. https://doi.org/10.3390/jcm9113538
APA StyleCwalinski, T., Polom, W., Marano, L., Roviello, G., D’Angelo, A., Cwalina, N., Matuszewski, M., Roviello, F., Jaskiewicz, J., & Polom, K. (2020). Methylene Blue—Current Knowledge, Fluorescent Properties, and Its Future Use. Journal of Clinical Medicine, 9(11), 3538. https://doi.org/10.3390/jcm9113538