Progress in Microneedle-Mediated Protein Delivery
Abstract
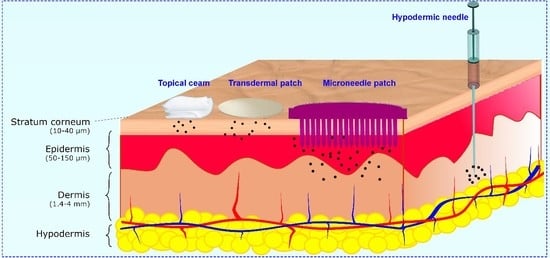
:1. Introduction
2. Fabrication Methods
2.1. Mold-Based Methods
2.2. Mold-Free Methods
3. Materials
4. Penetration and Mechanical Characterization Tests of Microneedles
5. Type of MNs Based on Their Structure and Release Profile
5.1. Hollow MNs
5.2. Coated MNs
5.3. Dissolvable Matrix MNs
5.4. Degradable Particle Embedded-MNs
5.5. Swellable MNs
5.6. Bio-responsive MNs
6. Characterization of MNs and Storage
6.1. Biomolecule Activity
6.2. Sterilization and Storage
7. Conclusions
8. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APC | antigen presenting cells |
| aPD1 | anti-programmed death-1 |
| BGLs | blood glucose levels |
| BGs | bioactive glass nanoparticles |
| BSA | bovine serum albumin |
| DDCs | dermal dendritic cells |
| DC | dendritic cell |
| DMNs | dissolvable microneedles |
| HA | hyaluronic acid |
| IF | interstitial fluid |
| iPEMs | immune polyelectrolyte multilayers |
| MBGs | mesoporous bioactive glasses |
| MNs | microneedles |
| MPs | microparticles |
| NPs | nanoparticles |
| OVA | ovalbumin |
| PBA | phenylboronic acid |
| PEG | poly (ethylene glycol) |
| PVA | polyvinyl acetate |
| PVP | polyvinylpyrrolidone |
| RF | radiofrequency |
| TDD | transdermal delivery |
| TFF | transverse failure force |
| TGA | thermal gravimetry analysis |
| W/O/W | water-in-oil-in-water emulsion method |
References
- Leader, B.; Baca, Q.J.; Golan, D.E. Protein therapeutics: A summary and pharmacological classification. Nat. Rev. Drug Discov. 2008, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Rawat, A.; Burgess, D.J. Parenteral Delivery of Peptides and Proteins, in Biodrug Delivery Systems; CRC Press: Boca Raton, FL, USA, 2016; pp. 66–84. [Google Scholar]
- Patel, A.; Cholkar, K.; Mitra, A.K. Recent developments in protein and peptide parenteral delivery approaches. Ther. Deliv. 2014, 5, 337–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundquist, P.; Artursson, P. Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev. 2016, 106, 256–276. [Google Scholar] [CrossRef]
- Al-Mayahy, M.H.; Sabri, A.H.; Rutland, C.S.; Holmes, A.; McKenna, J.; Marlow, M.; Scurr, D.J. Insight into imiquimod skin permeation and increased delivery using microneedle pre-treatment. Eur. J. Pharm. Biopharm. 2019, 139, 33–43. [Google Scholar] [CrossRef]
- Alkilani, A.; McCrudden, M.T.; Donnelly, R. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Yu, J.; Wen, D.; Kahkoska, A.R.; Gu, Z. Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev. 2018, 127, 106–118. [Google Scholar] [CrossRef]
- Takeuchi, A.; Nomoto, Y.; Watanabe, M.; Kimura, S.; Morimoto, Y.; Ueda, H. Application of microneedles to skin induces activation of epidermal Langerhans cells and dermal dendritic cells in mice. Biol. Pharm. Bull. 2016, 2016, b16-00113. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef]
- Shaikh, S.; Bhan, N.; Rodrigues, F.C.; Dathathri, E.; De, S.; Thakur, G. Microneedle Platform for Biomedical Applications, in Bioelectronics and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2019; pp. 421–441. [Google Scholar]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Shin, J.-H.; Seok, H.Y.; Kim, Y.-C. Biomedical applications of microneedles in therapeutics: Recent advancements and implications in drug delivery. Expert Opin. Drug Deliv. 2016, 13, 109–131. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, J.; Zhuang, J.; Xu, H.; Liu, Y.; Wu, D. Microneedle System for Transdermal Drug and Vaccine Delivery: Devices, Safety, and Prospects. Dose Response 2019, 17, 1559325819878585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larraneta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Pastor, Y.; Larrañeta, E.; Erhard, Á.; Quincoceset, G.; Peñuelas, I.; Irache, J.M.; Donnelly, R.; Gamazo, C. Dissolving Microneedles for Intradermal Vaccination against Shigellosis. Vaccines 2019, 7, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juster, H.; van der Aar, B.; de Brouwer, H. A review on microfabrication of thermoplastic polymer-based microneedle arrays. Polym. Eng. Sci. 2019, 59, 877–890. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, S.P.; Murthy, N.; Prausnitz, M.R. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Adv. Mater. 2008, 20, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Nejad, H.R.; Sadeqi, A.; Kiaee, G.; Sonkusale, S. Low-cost and cleanroom-free fabrication of microneedles. Microsyst. Nanoeng. 2018, 4, 17073. [Google Scholar] [CrossRef]
- Demir, Y.K.; Akan, Z.; Kerimoglu, O. Characterization of polymeric microneedle arrays for transdermal drug delivery. PLoS ONE 2013, 8, e77289. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Park, S.C.; Rizal, B.; Guanes, G.; Beak, S.-K.; Park, J.-H.; Betz, A.R.; Choi, S.-O. Fabrication of circular obelisk-type multilayer microneedles using micro-milling and spray deposition. Front. Bioeng. Biotechnol. 2018, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Krieger, K.J.; Bertollo, N.; Dangol, M.; Sheridan, J.T.; Lowery, M.M.; O’Cearbhaill, E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst. Nanoeng. 2019, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.R.; Procopio, A.T. Low cost additive manufacturing of microneedle masters. 3D Print. Med. 2019, 5, 2. [Google Scholar] [CrossRef]
- Andersen, T.E.; Andersen, A.J.; Petersen, R.S.; Nielsen, L.H.; Keller, S.S. Drug loaded biodegradable polymer microneedles fabricated by hot embossing. Microelectron. Eng. 2018, 195, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wu, D.; Liu, Y.; Yang, Z.; Gou, P. Rapid fabrication of micro structure on polypropylene by plate to plate isothermal hot embossing method. Polym. Eng. Sci. 2018, 58, 952–960. [Google Scholar]
- Zhuang, J.; Wu, D.-M.; Xu, H.; Huang, Y.; Liu, Y.; Sun, J.-Y. Edge Effect in Hot Embossing and its Influence on Global Pattern Replication of Polymer-Based Microneedles. Int. Polym. Process. 2019, 34, 231–238. [Google Scholar] [CrossRef]
- Janphuang, P.; Laebua, M.; Srihung, C.; Taweewat, P.; Sirichalarmkul, A.; Sukjantha, K.; Promasawat, N.; Leuasoongnoen, P.; Suhachiaraphan, S.; Phimol, K.; et al. Polymer based microneedle patch fabricated using microinjection moulding. In Proceedings of the MATEC Web of Conferences, Phuket, Thailand, 4–7 July 2018; EDP Sciences: Les Ulis, France, 2018. [Google Scholar]
- Ono, A.; Azukizawa, H.; Ito, S.; Nakamura, Y.; Asada, H.; Quan, Y.-S.; Kamiyama, F.; Katayama, I.; Hirobe, S.; Okada, N. Development of novel double-decker microneedle patches for transcutaneous vaccine delivery. Int. J. Pharm. 2017, 532, 374–383. [Google Scholar] [CrossRef]
- Yung, K.L.; Xu, Y.; Kang, C.; Liu, H.; Tam, K.F.; Ko, S.M.; Kwan, F.Y.; Lee, T.M.H. Sharp tipped plastic hollow microneedle array by microinjection moulding. J. Micromech. Microeng. 2011, 22, 015016. [Google Scholar] [CrossRef]
- Yang, S.; Feng, Y.; Zhang, L.; Chen, N.; Yuan, W.; Jin, T. A scalable fabrication process of polymer microneedles. Int. J. Nanomed. 2012, 7, 1415. [Google Scholar]
- Mönkäre, J.; Nejadnik, M.R.; Baccouche, K.; Romejin, S.; Jiskoot, W.; Bouwstra, J. IgG-loaded hyaluronan-based dissolving microneedles for intradermal protein delivery. J. Control. Release 2015, 218, 53–62. [Google Scholar] [CrossRef]
- Chen, M.-C.; Ling, M.-H.; Kusuma, S.J. Poly-γ-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin. Acta Biomater. 2015, 24, 106–116. [Google Scholar] [CrossRef]
- Park, Y.-H.; Ha, S.K.; Choi, I.; Kim, K.S.; Park, J.; Choi, N.; Kim, B.; Sung, J.H. Fabrication of degradable carboxymethyl cellulose (CMC) microneedle with laser writing and replica molding process for enhancement of transdermal drug delivery. Biotechnol. Bioprocess Eng. 2016, 21, 110–118. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, S.C.; Choi, S.-O. Dual-nozzle spray deposition process for improving the stability of proteins in polymer microneedles. RSC Adv. 2017, 7, 55350–55359. [Google Scholar] [CrossRef] [Green Version]
- McGrath, M.G.; Vucen, S.; Vrdoljak, A.; Kelly, A.; O’Mahony, C.; Crean, A.M.; Moore, A. Production of dissolvable microneedles using an atomised spray process: Effect of microneedle composition on skin penetration. Eur. J. Pharm. Biopharm. 2014, 86, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Park, J.; Chu, G.S.; Kim, K.S.; Sung, J.H.; Kim, B. Transdermal delivery of cosmetic ingredients using dissolving polymer microneedle arrays. Biotechnol. Bioprocess Eng. 2015, 20, 543–549. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J.D.; Lee, C.Y.; Her, S.; Jung, H. A high-capacity, hybrid electro-microneedle for in-situ cutaneous gene transfer. Biomaterials 2011, 32, 7705–7710. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Kim, M.; Yang, H.; Lee, K.; Jung, H. Droplet-born air blowing: Novel dissolving microneedle fabrication. J. Control. Release 2013, 170, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yang, H.; Kim, S.; Lee, C.; Jung, H. The troy microneedle: A rapidly separating, dissolving microneedle formed by Cyclic Contact and Drying on the Pillar (CCDP). PLoS ONE 2015, 10, e0136513. [Google Scholar] [CrossRef]
- Ruggiero, F.; Vecchione, R.; Bhowmick, S.; Coppola, G.; Coppola, S.; Esposito, E.; Lettera, V.; Ferraro, P.; Netti, P.A. Electro-drawn polymer microneedle arrays with controlled shape and dimension. Sens. Actuators B Chem. 2018, 255, 1553–1560. [Google Scholar] [CrossRef]
- Vecchione, R.; Coppola, S.; Esposito, E.; Casale, C.; Vespini, V.; Grilli, S.; Ferraro, P.; Netti, P.A. Electro-drawn drug-loaded biodegradable polymer microneedles as a viable route to hypodermic injection. Adv. Funct. Mater. 2014, 24, 3515–3523. [Google Scholar] [CrossRef]
- Luzuriaga, M.A.; Berry, D.R.; Reagan, J.C.; Smaldone, R.A.; Gassensmith, J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip 2018, 18, 1223–1230. [Google Scholar] [CrossRef]
- Economidou, S.N.; Lamprou, D.A.; Douroumis, D. 3D printing applications for transdermal drug delivery. Int. J. Pharm. 2018, 544, 415–424. [Google Scholar] [CrossRef]
- Lu, Y.; Mantha, S.N.; Crowder, D.C.; Chinchilla, S.; Shah, K.N.; Yun, Y.H.; Wicker, R.B.; Choi, J.-W. Microstereolithography and characterization of poly (propylene fumarate)-based drug-loaded microneedle arrays. Biofabrication 2015, 7, 045001. [Google Scholar] [CrossRef]
- Lim, S.H.; Ng, J.Y.; Kang, L. Three-dimensional printing of a microneedle array on personalized curved surfaces for dual-pronged treatment of trigger finger. Biofabrication 2017, 9, 015010. [Google Scholar] [CrossRef] [PubMed]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moussi, K.; Bukhamsin, A.; Hidalgo, T.; Kosel, J. Biocompatible 3D Printed Microneedles for Transdermal, Intradermal, and Percutaneous Applications. Adv. Eng. Mater. 2019, 2019, 1901358. [Google Scholar]
- Moussi, K.; Bukhamsin, A.; Kosel, J. ImplanTable 3D Printed Drug Delivery System. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems Eurosensors XXXIII (TRANSDUCERS EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019. [Google Scholar]
- Yeung, C.; Chen, S.; King, B.; Lin, H.; King, K.; Akhtar, F.; Diaz, G.; Wang, B.; Zhu, J.; Sun, W.; et al. A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery. Biomicrofluidics 2019, 13, 064125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Larrañeta, E.; McCrudden Wiley, M.T.C. Microneedles for Drug and Vaccine Delivery and Patient Monitoring; Wiley Online Library: Hoboken, NJ, USA, 2018. [Google Scholar]
- Zhu, M.W.; Li, H.W.; Chen, X.L.; Tang, Y.F.; Lu, M.H.; Chen, Y.F. Silica needle template fabrication of metal hollow microneedle arrays. J. Micromech. Microeng. 2009, 19, 115010. [Google Scholar] [CrossRef]
- Gittard, S.D.; Narayan, R.J.; Jin, C.; Ovsianikov, A.; Chichkov, B.N.; Monteiro-Riviere, N.A.; Stafslien, S.; Chisholm, B. Pulsed laser deposition of antimicrobial silver coating on Ormocer® microneedles. Biofabrication 2009, 1, 041001. [Google Scholar] [CrossRef] [Green Version]
- Larrañeta, E.; Singh, T.R.R. Microneedle Manufacturing and Testing. In Microneedles for Drug and Vacccine Delivery and Patient Monitoring; Ryan, F.D., Ed.; Jhon Wiley Sons: Hoboken, NJ, USA, 2018; pp. 1–70. [Google Scholar]
- Li, G.; Badkar, A.; Nerna, S.; Kolli, C.S.; Banga, A.K. In vitro transdermal delivery of therapeutic antibodies using maltose microneedles. Int. J. Pharm. 2009, 368, 109–115. [Google Scholar] [CrossRef]
- Miyano, T.; Tobinaga, Y.; Kanno, T.; Matsuzaki, Y.; Takeda, H.; Wakui, M.; Hanada, K. Sugar micro needles as transdermic drug delivery system. Biomed. Microdevices 2005, 7, 185–188. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Sala, F.D.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 2019, 115023. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Sala, F.D.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110195. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Makvandi, P.; Tay, F.R. Recent progress in the industrial and biomedical applications of tragacanth gum. Carbohydr. Polym. 2019, 212, 450–467. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Makvandi, P.; Borzacchiello, A.; Tay, F.R.; Ashtari, B.; Padil, V.V.T. Antimicrobial gum bio-based nanocomposites and their industrial and biomedical applications. Chem. Commun. 2019, 55, 14871–14885. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, S.; Izumri, H.; Isono, Y.; Fukuda, M.; Ogawa, H. Laser fabrication of high aspect ratio thin holes on biodegradable polymer and its application to a microneedle. Sens. Actuators A Phys. 2007, 139, 293–302. [Google Scholar] [CrossRef]
- Pérennès, F.; Marmiroli, B.; Matteucci, M.; Tormen, M.; Vaccari, L.; Fabrizio, E.D. Sharp beveled tip hollow microneedle arrays fabricated by LIGA and 3D soft lithography with polyvinyl alcohol. J. Micromech. Microeng. 2006, 16, 473. [Google Scholar] [CrossRef]
- Battisti, M.; Vecchione, R.; Casale, C.; Pennachio, F.A.; Lettera, V.; Jamaledin, R.; Profeta, M.; Di Natale, C.; Imparato, G.; Urciuolo, F.; et al. Non-invasive production of multi-compartmental biodegradable polymer microneedles for controlled intradermal drug release of labile molecules. Front. Bioeng. Biotechnol. 2019, 7, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, R.F.; Majithiya, R.; Singh, T.R.R.; Morrow, D.I.J.; Garland, M.J.; Demir, Y.K.; Migalska, K.; Ryan, E.; Gillen, D.; Scott, C.J.; et al. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm. Res. 2011, 28, 41–57. [Google Scholar] [CrossRef] [Green Version]
- Cahill, E.M.; O’Cearbhaill, E.D. Toward biofunctional microneedles for stimulus responsive drug delivery. Bioconjugate Chem. 2015, 26, 1289–1296. [Google Scholar] [CrossRef]
- Giusti, F.; Martella, A.; Bertoni, L.; Seidenri, S. Skin barrier, hydration, and pH of the skin of infants under 2 years of age. Pediatr. Dermatol. 2001, 18, 93–96. [Google Scholar] [CrossRef]
- Stücker, M.; Struk, A.; Altmeyer, P.; Herde, M.; Baumgärtl, H.; Lübbers, D.W. The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and epidermis. J. Physiol. 2002, 538, 985–994. [Google Scholar] [CrossRef]
- Park, J.-H.; Allen, M.G.; Prausnitz, M.R. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J. Control. Release 2005, 104, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-M.; Todo, H.; Sugibayashi, K. Enhancement of skin permeation of high molecular compounds by a combination of microneedle pretreatment and iontophoresis. J. Control. Release 2007, 118, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Miller, P.R.; Boehm, R.D.; Ovsiankov, A.; Chickov, B.N.; Heiser, J.; Gordon, J.; Monteiro-Riviere, N.A.; Narayan, R.J. Multiphoton microscopy of transdermal quantum dot delivery using two photon polymerization-fabricated polymer microneedles. Faraday Discuss. 2011, 149, 171–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutton, R.E.M.; Moore, J.; Larrañeta, E.; Ligett, S.; Woolfson, A.D.; Donnelly, R.F. Microneedle characterisation: The need for universal acceptance criteria and GMP specifications when moving towards commercialisation. Drug Deliv. Transl. Res. 2015, 5, 313–331. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, R.F.; Garland, M.J.; Morrow, D.I.J.; Migalska, K.; Singh, T.R.R.; Majithiya, R.; Woolfson, A.D. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. J. Control. Release 2010, 147, 333–341. [Google Scholar] [CrossRef]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef]
- Niu, L.; Chu, L.Y.; Burton, S.A.; Hansen, K.J.; Panyam, J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J. Control. Release 2019, 294, 268–278. [Google Scholar] [CrossRef]
- Du, G.; Hathout, R.M.; Nasr, M.; Nejadnik, M.R.; Tu, J.; Koning, R.I.; Koster, A.J.; Slütter, B.; Kros, A.; Jiskoot, W.; et al. Intradermal vaccination with hollow microneedles: A comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J. Control. Release 2017, 266, 109–118. [Google Scholar] [CrossRef]
- Du, G.; Leone, M.; Romeijn, S.; Kersten, G.; Jiskoot, W.; Bouwstra, J.A. Immunogenicity of diphtheria toxoid and poly (I: C) loaded cationic liposomes after hollow microneedle-mediated intradermal injection in mice. Int. J. Pharm. 2018, 547, 250–257. [Google Scholar] [CrossRef]
- Dragicevic, N.; Maibach, H.I. Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement; Springer: New York, NY, USA, 2017. [Google Scholar]
- Wang, P.M.; Cornwell, M.; Hill, J.; Prausnitz, M.R. Precise microinjection into skin using hollow microneedles. J. Investig. Dermatol. 2006, 126, 1080–1087. [Google Scholar] [CrossRef] [Green Version]
- Sabri, A.H.; Ogilvie, J.; Abdulhamid, K.; Shpadaruk, V.; McKenna, J.; Segal, J.; Scurr, D.J.; Marlow, M. Expanding the applications of microneedles in dermatology. Eur. J. Pharm. Biopharm. 2019, 140, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Martanto, W.; Moore, J.S.; Kashlan, O.; Kamath, R.; Wang, P.M.; O’Neal, J.M.; Prausnitz, M.R. Microinfusion using hollow microneedles. Pharm. Res. 2006, 23, 104–113. [Google Scholar] [CrossRef] [PubMed]
- DeMuth, P.C.; Li, A.V.; Abbink, P.; Liu, J.; Li, H.; Stanley, K.A.; Smith, K.M.; Lavine, C.L.; Seaman, M.S.; Kramer, J.A.; et al. Vaccine delivery with microneedle skin patches in nonhuman primates. Nat. Biotechnol. 2013, 31, 1082–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Chen, B.Z.; Wang, Q.L.; Jin, X.; Guo, X.D. Fabrication of coated polymer microneedles for transdermal drug delivery. J. Control. Release 2017, 265, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Ameri, M.; Kadkhodayan, M.; Nguyen, J.; Bravo, J.A.; Su, R.; Chan, K.; Samiee, A.; Daddona, P.E. Human growth hormone delivery with a microneedle transdermal system: Preclinical formulation, stability, delivery and PK of therapeutically relevant doses. Pharmaceutics 2014, 6, 220–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusamori, K.; Katsumi, H.; Sakai, R.; Hayashi, R.; Hirai, Y.; Tanaka, Y.; Hitomi, K.; Quan, Y.-S.; Kamiyama, F.; Yamada, K.; et al. Development of a drug-coated microneedle array and its application for transdermal delivery of interferon alpha. Biofabrication 2016, 8, 015006. [Google Scholar] [CrossRef] [PubMed]
- Daddona, P.E.; Matriano, J.A.; Mandema, J.; Maa, Y.-F. Parathyroid hormone (1-34)-coated microneedle patch system: Clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm. Res. 2011, 28, 159–165. [Google Scholar] [CrossRef]
- Koutsonanos, D.G.; Esser, E.S.; McMaster, S.R.; Kalluri, P.; Lee, J.-W.; Prausnitz, M.R.; Skountzou, I.; Denning, T.L.; Kohlmeier, J.E.; Compans, R.W. Enhanced immune responses by skin vaccination with influenza subunit vaccine in young hosts. Vaccine 2015, 33, 4675–4682. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Verbaan, F.J.; Bivas-Benita, M.; Bungener, L.; Huckriede, A.; van den Berg, D.J.; Kersten, G.; Bouwstra, J.A. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J. Control. Release 2009, 136, 71–78. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Quan, F.-S.; Compans, R.W.; Kang, S.-M.; Prausnitz, M.R. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J. Control. Release 2010, 142, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Alarcon, J.B.; Hartley, A.W.; Harvey, N.G.; Mikszta, J.A. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin. Vaccine Immunol. 2007, 14, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnou, R.; Icardi, G.; De Decker, M.; Ambrozaitis, A.; Kazek, M.-P.; Weber, F.; Damme, P.V. Intradermal influenza vaccine for older adults: A randomized controlled multicenter phase III study. Vaccine 2009, 27, 7304–7312. [Google Scholar] [CrossRef] [PubMed]
- Beran, J.; Ambrozaitis, A.; Laiskonis, A.; Mickuviene, N.; Bacart, P.; Calozet, Y.; Demanet, E.; Heijmans, S.; Belle, P.V.; Weber, F.; et al. Intradermal influenza vaccination of healthy adults using a new microinjection system: A 3-year randomised controlled safety and immunogenicity trial. BMC Med. 2009, 7, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.-J.; Bondy, B.J.; Yoo, D.G.; Compans, R.W.; Kang, S.-M.; Prausnitz, M. Stability of whole inactivated influenza virus vaccine during coating onto metal microneedles. J. Control. Release 2013, 166, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.-J.; Yoo, D.-G.; Bondy, B.J.; Quan, F.-S.; Compans, R.W.; Kang, S.-M.; Prausnitz, M.R. Stability of influenza vaccine coated onto microneedles. Biomaterials 2012, 33, 3756–3769. [Google Scholar] [CrossRef] [Green Version]
- Fernando, G.J.P.; Chen, X.; Primiero, C.A.; Yukiko, S.R.; Fairmaid, E.J.; Corbett, H.J.; Frazer, I.H.; Brown, L.E.; Kendall, M.A. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. J. Control. Release 2012, 159, 215–221. [Google Scholar] [CrossRef]
- Fernando, G.J.; Chen, X.; Prow, T.W.; Crichton, M.L.; Fairmaid, E.J.; Roberts, M.S.; Frazer, I.H.; Brown, L.E.; Kendall, M.A.F. Potent immunity to low doses of influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PLoS ONE 2010, 5, e10266. [Google Scholar] [CrossRef]
- Holland, D.; Booy, R.; De Looze, F.; Eizenberg, P.; McDonald, J.; Karrasch, J.; McKeirnan, M.; Salem, H.; Mills, G.; Reid, J.; et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: A randomized controlled trial. J. Infect. Dis. 2008, 198, 650–658. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-C.; Quantity, F.-S.; Compans, R.W.; Kang, S.-M.; Prausnitz, M.R. Stability kinetics of influenza vaccine coated onto microneedles during drying and storage. Pharm. Res. 2011, 28, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-C.; Quan, F.-S.; Yoo, D.-G.; Compans, R.W.; Kang, S.-M.; Prausnitz, M.R. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J. Infect. Dis. 2010, 201, 190–198. [Google Scholar] [CrossRef]
- Kommareddy, S.; Baudner, B.C.; Bonificio, A.; Gallorini, S.; Palladino, G.; Determan, A.S.; Dohmeier, D.M.; Kroells, K.D.; Sternjohn, J.R.; Singh, M.; et al. Influenza subunit vaccine coated microneedle patches elicit comparable immune responses to intramuscular injection in guinea pigs. Vaccine 2013, 31, 3435–3441. [Google Scholar] [CrossRef] [PubMed]
- Kommareddy, S.; Baudner, B.C.; Oh, S.; Kwon, S.-Y.; Singh, M.; O’Hagan, D.T. Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. J. Pharm. Sci. 2012, 101, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Levin, Y.; Kochba, E.; Kenney, R. Clinical evaluation of a novel microneedle device for intradermal delivery of an influenza vaccine: Are all delivery methods the same? Vaccine 2014, 32, 4249–4252. [Google Scholar] [CrossRef] [PubMed]
- Weldon, W.C.; Zarnitsyn, V.G.; Esser, E.S.; Taherbhai, M.T.; Koutsonanos, D.G.; Vassilieva, E.V.; Skountzou, I.; Prausnitz, M.P.; Compans, R.W. Effect of adjuvants on responses to skin immunization by microneedles coated with influenza subunit vaccine. PLoS ONE 2012, 7, e41501. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Gammon, J.M.; Tostanoski, L.H.; Chiu, Y.-C.; Jewell, C.M. In vivo expansion of melanoma-specific T cells using microneedle arrays coated with immune-polyelectrolyte multilayers. ACS Biomater. Sci. Eng. 2016, 3, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, S.-O.; Felner, E.I.; Prausnitz, M.R. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small 2011, 7, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Park, J.-H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, S.P.; Koutsonanos, D.G.; Martin, M.D.P.; Lee, J.W.; Zarnitsyn, V.; Choi, S.-O.; Murthy, N.; Compans, R.W.; Skountzou, I.; Prausnitz, M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010, 16, 915. [Google Scholar] [CrossRef] [PubMed]
- Raphael, A.P.; Prow, T.W.; Crichton, M.L.; Chen, X.; Fernando, G.J.P.; Kendall, M.A.F. Targeted, needle-free vaccinations in skin using multilayered, densely-packed dissolving microprojection arrays. Small 2010, 6, 1785–1793. [Google Scholar] [CrossRef]
- Matsuo, K.; Hirobe, S.; Yokota, Y.; Ayabe, Y.; Seto, M.; Quan, Y.-S.; Kamiyama, F.; Tougan, T.; Horii, T.; Mukai, Y.; et al. Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza. J. Control. Release 2012, 160, 495–501. [Google Scholar] [CrossRef]
- Ling, M.-H.; Chen, M.-C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013, 9, 8952–8961. [Google Scholar] [CrossRef]
- Matsuo, K.; Okamoto, H.; Kawai, Y.; Quan, Y.-S.; Kamiyama, F.; Hirobe, S.; Okada, N.; Nakagawa, S. Vaccine efficacy of transcutaneous immunization with amyloid β using a dissolving microneedle array in a mouse model of Alzheimer’s disease. J. Neuroimmunol. 2014, 266, 1–11. [Google Scholar] [CrossRef]
- Kolluru, C.; Gomaa, Y.; Prausnitz, M.R. Development of a thermostable microneedle patch for polio vaccination. Drug Deliv. Transl. Res. 2019, 9, 192–203. [Google Scholar] [CrossRef] [Green Version]
- Hong, X.; Wei, L.; Wu, F.; Wu, Z.; Chen, L.; Liu, Z.; Yuan, W. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des. Dev. Ther. 2013, 7, 945–952. [Google Scholar]
- Cohen-Sela, E.; Chorny, M.; Koroukhov, N.; Danenberg, H.D.; Golomb, G. A new double emulsion solvent diffusion technique for encapsulating hydrophilic molecules in PLGA nanoparticles. J. Control. Release 2009, 133, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Sah, E.; Sah, H. Recent trends in preparation of poly (lactide-co-glycolide) nanoparticles by mixing polymeric organic solution with antisolvent. J. Nanomater. 2015, 16, 61. [Google Scholar] [CrossRef] [Green Version]
- Walters, A.A.; Somavarapu, S.; Riitho, V.; Stewart, G.R.; Charleston, B.; Steinbach, F.; Graham, S.P. Assessment of the enhancement of PLGA nanoparticle uptake by dendritic cells through the addition of natural receptor ligands and monoclonal antibody. Vaccine 2015, 33, 6588–6595. [Google Scholar] [CrossRef]
- Zaric, M.; Lyuomska, O.; Touzelet, O.; Poux, C.; AI-Zahrani, S.; Fay, F.; Wallace, L.; Terhorst, D.; Malissen, B.; Henri, S.; et al. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-D,L-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano 2013, 7, 2042–2055. [Google Scholar] [CrossRef] [PubMed]
- Mazzara, J.M.; Ochyl, L.J.; Hong, J.K.Y.; Moon, J.J.; Prausnitz, M.R.; Schwendeman, S.P. Self-healing encapsulation and controlled release of vaccine antigens from PLGA microparticles delivered by microneedle patches. Bioeng. Transl. Med. 2019, 4, 116–128. [Google Scholar] [CrossRef] [Green Version]
- DeMuth, P.C.; Garcia-Beltran, W.F.; Ai-Ling, M.L.; Hammond, P.T.; Irvine, D.J. Composite dissolving microneedles for coordinated control of antigen and adjuvant delivery kinetics in transcutaneous vaccination. Adv. Funct. Mater. 2013, 23, 161–172. [Google Scholar] [CrossRef] [Green Version]
- DeMuth, P.C.; Min, Y.; Irvine, D.J.; Hammond, P.T. Implantable silk composite microneedles for programmable vaccine release kinetics and enhanced immunogenicity in transcutaneous immunization. Adv. Healthc. Mater. 2014, 3, 47–58. [Google Scholar] [CrossRef]
- Migdadi, E.M.; Courtenay, A.J.; Tekko, I.A.; McCrudden, M.T.C.; Kearney, M.-C.; McAlister, E.; McCarthy, H.O.; Donnelly, R.F. Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J. Control. Release 2018, 285, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Wu, Z.; Chen, L.; Wu, F.; Wei, L.; Yuan, W. Hydrogel microneedle arrays for transdermal drug delivery. Nano Micro Lett. 2014, 6, 191–199. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.T.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-Forming Microneedle Arrays for Enhanced Transdermal Drug Delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Liu, S.; Feng, W. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, F.; Liu, J.; Fan, G.; William, W.; Zhu, H.; Jin, T. Phase-transition microneedle patches for efficient and accurate transdermal delivery of insulin. Adv. Funct. Mater. 2015, 25, 4633–4641. [Google Scholar] [CrossRef]
- Chang, H.; Zheng, M.; Yu, X.; Than, A.; Seeni, R.Z.; Kang, R.; Tian, J.; Khanh, D.P.; Liu, L.; Chen, P.; et al. A swellable microneedle patch to rapidly extract skin interstitial fluid for timely metabolic analysis. Adv. Mater. 2017, 29, 1702243. [Google Scholar] [CrossRef]
- Seong, K.-Y.; Seo, M.-S.; Hwang, D.Y.; O’Cearbhail, E.D.; Sreenan, S.; Karp, J.M.; Yang, S.Y. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J. Control. Release 2017, 265, 48–56. [Google Scholar] [CrossRef]
- Courtenay, A.J.; McCrudden, M.T.C.; McAvoy, K.J.; McCarthy, H.O.; Donnelly, R.F. Microneedle-Mediated Transdermal Delivery of Bevacizumab. Mol. Pharm. 2018, 15, 3545–3556. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 1–17. [Google Scholar] [CrossRef]
- Baghban Taraghdari, Z.; Imani, R.; Mohabatpour, F. A Review on Bioengineering Approaches to Insulin Delivery: A Pharmaceutical and Engineering Perspective. Macromol. Biosci. 2019, 19, 1800458. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Yu, J.; Kahkoska, A.R.; Buse, J.B.; Gu, Z. Glucose-Responsive Insulin and Delivery Systems: Innovation and Translation. Adv. Mater. 2019, 2019, 1902004. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, Y.; Yu, J.; Kahkoska, A.R.; Zhang, X.; Wang, C.; Sun, W.; Corder, R.D.; Chen, Z.; Khan, S.A.; et al. Core–Shell Microneedle Gel for Self-Regulated Insulin Delivery. ACS Nano 2018, 12, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, X.; Liu, Y.; Huang, F.; Wu, G.; Liu, Y.; Zhang, Z.; Ding, Y.; Lv, J.; Ma, R.; et al. Glucose and H 2 O 2 dual-sensitive nanogels for enhanced glucose-responsive insulin delivery. Nanoscale 2019, 11, 9163–9175. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Matsumoto, H.; Moro-oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Smart Microneedle Fabricated with Silk Fibroin Combined Semi-interpenetrating Network Hydrogel for Glucose-Responsive Insulin Delivery. ACS Biomater. Sci. Eng. 2019, 5, 5781–5789. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, B.; Zhu, J.; Zhang, Y.; Liu, T.; Cao, C. Polymer microneedles integrated with glucose-responsive mesoporous bioactive glass nanoparticles for transdermal delivery of insulin. Biomed. Phys. Eng. Express 2019, 5, 045038. [Google Scholar] [CrossRef]
- Xu, B.; Cao, Q.; Zhang, Y.; Yu, W.; Zhu, J.; Liu, D.; Jiang, G. Microneedles Integrated with ZnO Quantum-Dot-Capped Mesoporous Bioactive Glasses for Glucose-Mediated Insulin Delivery. ACS Biomater. Sci. Eng. 2018, 4, 2473–2483. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, J.; Wang, C.; Nguyen, N.-Y.; Walker, G.M.; Buse, J.B.; Gu, Z. Microneedles integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery. Adv. Mater. 2016, 28, 3115–3121. [Google Scholar] [CrossRef] [Green Version]
- Mofidfar, M.; Prausnitz, M.R. Design, Structure, Material strength and Release Profile of Dissolvable Microneedle Patches. In Proceedings of the Society for Biomaterials 2018 Annual Meeting and Exposition, Atlanta, GA, USA, 11–14 April 2018. [Google Scholar]
- Mistilis, M.J.; Bommarius, A.S.; Prausnitz, M.R. Development of a thermostable microneedle patch for influenza vaccination. J. Pharm. Sci. 2015, 104, 740–749. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019, 4, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Panyam, J.; Dali, M.M.; Sahoo, S.K.; Ma, W.; Chakravarthi, S.C.; Amidon, G.L.; Levy, R.J.; Labhasetwar, V. Polymer degradation and in vitro release of a model protein from poly (D,L-lactide-co-glycolide) nano-and microparticles. J. Control. Release 2003, 92, 173–187. [Google Scholar] [CrossRef]
- Xie, J.; Wang, C.H. Encapsulation of proteins in biodegradable polymeric microparticles using electrospray in the Taylor cone-jet mode. Biotechnol. Bioeng. 2007, 97, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chan, S.Y.; Lee, W.G.; Kang, L. Microfabricated particulate drug-delivery systems. Biotechnol. J. 2011, 6, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Angelov, B.; Mutafchieva, R.; Lesieur, S.; Couvreur, P. Self-assembled multicompartment liquid crystalline lipid carriers for protein, peptide, and nucleic acid drug delivery. Acc. Chem. Res. 2010, 44, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, Y.; Hagiwara, E.; Saeki, A.; Sugioka, N.; Takada, K. Feasibility of microneedles for percutaneous absorption of insulin. Eur. J. Pharm. Sci. 2006, 29, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.Y.; Lee, J.; Kim, B.J.; Joo, K.I.; Kim, K.H.; Lim, G.; Cha, H.J. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials 2019, 222, 119439. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.; Duan, Y.; Niu, Y.; Gu, H.; Zhao, Z.; Zhang, S.; Yang, Y.; Wang, X.; Gao, Y.; et al. Transcutaneous immunization of recombinant Staphylococcal enterotoxin B protein using a dissolving microneedle provides potent protection against lethal enterotoxin challenge. Vaccine 2019, 37, 3810–3819. [Google Scholar] [CrossRef]
- Kochhar, J.S.; Zou, S.; Chan, S.Y.; Kang, L. Protein encapsulation in polymeric microneedles by photolithography. Int. J. Nanomed. 2012, 7, 3143. [Google Scholar]
- Donnelly, R.F.; Singh, T.R.R.; Tunney, M.M.; Morrow, D.I.J.; McCarron, P.A.; O’Mahony, C.; Woolfson, A.D. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm. Res. 2009, 26, 2513–2522. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, A.M.; Cordeiro, A.S.; Donnelly, R.F. Technology update: Dissolvable microneedle patches for vaccine delivery. Med. Devices 2019, 12, 379. [Google Scholar] [CrossRef] [Green Version]
- Li, W.Z.; Huo, M.-R.; Zhou, J.-P.; Zhou, Y.-Q.; Hao, B.-H.; Liu, T.; Zhang, Y. Super-short solid silicon microneedles for transdermal drug delivery applications. Int. J. Pharm. 2010, 389, 122–129. [Google Scholar]
- Makvandi, P.; Corcione, C.E.; Paladini, F.; Gallo, A.L.; Montagna, F.; Jamaledin, R.; Pollini, M.; Maffezzoli, A. Antimicrobial modified hydroxyapatite composite dental bite by stereolithography. Polym. Adv. Technol. 2018, 29, 364–371. [Google Scholar] [CrossRef]
- Makvandi, P.; Ghaemy, M.; Ghadiri, A.A.; Mohseni, M. Photocurable, antimicrobial quaternary ammonium–modified nanosilica. J. Dent. Res. 2015, 94, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Gu, J.T.; Zare, E.N.; Ashtari, B.; Moeini, A.; Tay, F.R.; Niu, L.-N. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2020, 101, 69–101. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, Y.; Nakagawa, T.; Quan, Y.S.; Kamiyama, F.; Hirobe, S.; Okada, N.; Nakagawa, S. Performance and Characteristics Evaluation of a Sodium Hyaluronate-Based Microneedle Patch for a Transcutaneous Drug Delivery System. Int. J. Pharm. 2012, 441, 570–579. [Google Scholar] [CrossRef]
- van Bergen, M. Cooperative RD Projects between Biotechnology Firms and Public Research Institutes: Determinants of Success from a Product Competitive Advantage Perspective. Ph.D. Thesis, Universität Koblenz-Landau, Koblenz, Germany, 2019. [Google Scholar]
- Patil, S.; Narvekar, A.; Puranik, A.; Jain, R.; Dandekar, P. Formulation of Therapeutic Proteins: Strategies for Developing Oral Protein Formulations. Innov. Dos. Forms Des. Dev. Early Stage 2019, 2019, 391–432. [Google Scholar]
- Frieden, C. Protein aggregation processes: In search of the mechanism. Protein Sci. 2007, 16, 2334–2344. [Google Scholar] [CrossRef]
- Di Natale, C.; Manna, S.L.; Malfitano, A.M.; Somma, S.D.; Florio, D.; Scognamiglio, P.L.; Novellino, E.; Netti, P.A.; Marasco, D. Structural insights into amyloid structures of the C-terminal region of nucleophosmin 1 in type A mutation of acute myeloid leukemia. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2019, 1867, 637–644. [Google Scholar] [CrossRef]
- Moisturising, U.N.; Customisable, H.; Mass, S. P08 P14 P19. ONdrugDelivery Magazine, 31 March 2015. Available online: https://multimedia.3m.com/mws/media/1080389O/ondrugdelivery-article-march-2015.pdf (accessed on 16 February 2020).
- Kim, M.; Jung, B.; Park, J.-H. Hydrogel swelling as a trigger to release biodegradable polymer microneedles in skin. Biomaterials 2012, 33, 668–678. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamaledin, R.; Di Natale, C.; Onesto, V.; Taraghdari, Z.B.; Zare, E.N.; Makvandi, P.; Vecchione, R.; Netti, P.A. Progress in Microneedle-Mediated Protein Delivery. J. Clin. Med. 2020, 9, 542. https://doi.org/10.3390/jcm9020542
Jamaledin R, Di Natale C, Onesto V, Taraghdari ZB, Zare EN, Makvandi P, Vecchione R, Netti PA. Progress in Microneedle-Mediated Protein Delivery. Journal of Clinical Medicine. 2020; 9(2):542. https://doi.org/10.3390/jcm9020542
Chicago/Turabian StyleJamaledin, Rezvan, Concetta Di Natale, Valentina Onesto, Zahra Baghban Taraghdari, Ehsan Nazarzadeh Zare, Pooyan Makvandi, Raffaele Vecchione, and Paolo Antonio Netti. 2020. "Progress in Microneedle-Mediated Protein Delivery" Journal of Clinical Medicine 9, no. 2: 542. https://doi.org/10.3390/jcm9020542
APA StyleJamaledin, R., Di Natale, C., Onesto, V., Taraghdari, Z. B., Zare, E. N., Makvandi, P., Vecchione, R., & Netti, P. A. (2020). Progress in Microneedle-Mediated Protein Delivery. Journal of Clinical Medicine, 9(2), 542. https://doi.org/10.3390/jcm9020542