Fractional Flow Reserve Derived from Coronary Computed Tomography Angiography Safely Defers Invasive Coronary Angiography in Patients with Stable Coronary Artery Disease
Abstract
:1. Introduction
2. Methods
2.1. Coronary Computed Tomography Angiography Acquisition and Analysis
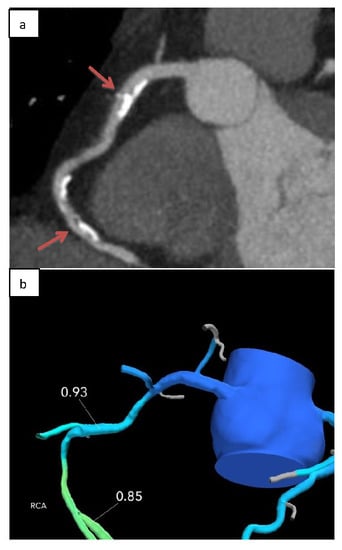
2.2. Computation of Fractional Flow Reserve from Coronary Computed Tomography Angiography
2.3. Diagnostic Invasive Coronary Angiography
2.4. Study End Points and Follow-Up
2.5. Statistical Analysis
3. Results
4. Discussion
- (1)
- FFRCT was feasible with a conclusive result in >90% of patients;
- (2)
- A diagnostic strategy of coronary CTA plus FFRCT was associated with less ICA in patients with CAD, compared to coronary CTA alone;
- (3)
- Among those who deferred ICA, there was no MACE after more than a one-year follow-up;
- (4)
- A high proportion of those who underwent ICA were revascularized, resulting in higher diagnostic ICA yield and more efficient utilization of catheterization lab resources.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CAD | coronary artery disease |
| CTA | computed tomography angiography |
| FFR | fractional flow reserve |
| FFRCT | fractional flow reserve derived from coronary computed tomography angiography datasets |
| ICA | invasive coronary angiogram |
| MACE | major adverse cardiac event |
| SPECT | single photon emission computed tomography |
References
- Patel, M.R.; Peterson, E.D.; Dai, D.; Brennan, J.M.; Redberg, R.F.; Anderson, H.V.; Brindis, R.G.; Douglas, P.S. Low diagnostic yield of elective coronary angiography. N. Engl. J. Med. 2010, 362, 886–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.R.; Dai, D.; Hernandez, A.F.; Douglas, P.S.; Messenger, J.; Garratt, K.N.; Maddox, T.M.; Peterson, E.D.; Roe, M.T. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am. Heart J. 2014, 167, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.C. President’s page: Coronary CT angiography as a gatekeeper to the catheterization laboratory. J. Cardiovasc. Comput. Tomogr. 2014, 8, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Vavalle, J.P.; Shen, L.; Broderick, S.; Shaw, L.K.; Douglas, P.S. Effect of the Presence and Type of Angina on Cardiovascular Events in Patients Without Known Coronary Artery Disease Referred for Elective Coronary Angiography. JAMA Cardiol. 2016, 1, 232–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budoff, M.J.; Dowe, D.; Jollis, J.G.; Gitter, M.; Sutherland, J.; Halamert, E.; Scherer, M.; Bellinger, R.; Martin, A.; Benton, R.; et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter ACCURACY (Assessment by Coro. J. Am. Coll. Cardiol. 2008, 52, 1724–1732. [Google Scholar] [CrossRef] [Green Version]
- Meijboom, W.B.; Meijs, M.F.L.; Schuijf, J.D.; Cramer, M.J.; Mollet, N.R.; van Mieghem, C.A.G.; Nieman, K.; van Werkhoven, J.M.; Pundziute, G.; Weustink, A.C.; et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: A prospective, multicenter, multivendor study. J. Am. Coll. Cardiol. 2008, 52, 2135–2144. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.M.; Rochitte, C.E.; Dewey, M.; Arbab-Zadeh, A.; Niinuma, H.; Gottlieb, I.; Paul, N.; Clouse, M.E.; Shapiro, E.P.; Hoe, J.; et al. Diagnostic performance of coronary angiography by 64-row CT. N. Engl. J. Med. 2008, 359, 2324–2336. [Google Scholar] [CrossRef] [Green Version]
- Neglia, D.; Rovai, D.; Caselli, C.; Pietila, M.; Teresinska, A.; Aguadé-Bruix, S.; Pizzi, M.N.; Todiere, G.; Gimelli, A.; Schroeder, S.; et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ. Cardiovasc. Imaging 2015, 8, e002179. [Google Scholar] [CrossRef] [Green Version]
- Toth, G.; Hamilos, M.; Pyxaras, S.; Mangiacapra, F.; Nelis, O.; De Vroey, F.; Di Serafino, L.; Muller, O.; Van Mieghem, C.; Wyffels, E.; et al. Evolving concepts of angiogram: Fractional flow reserve discordances in 4000 coronary stenoses. Eur. Heart J. 2014, 35, 2831–2838. [Google Scholar] [CrossRef] [Green Version]
- Meijboom, W.B.; Van Mieghem, C.A.G.; van Pelt, N.; Weustink, A.; Pugliese, F.; Mollet, N.R.; Boersma, E.; Regar, E.; van Geuns, T.; de Jaegere, P.; et al. Comprehensive Assessment of Coronary Artery Stenoses. J. Am. Coll. Cardiol. 2008, 52, 636–643. [Google Scholar] [CrossRef] [Green Version]
- Tonino, P.A.L.; Fearon, W.F.; De Bruyne, B.; Oldroyd, K.G.; Leesar, M.A.; Ver Lee, P.N.; Maccarthy, P.A.; Van’t Veer, M.; Pijls, N.H. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J. Am. Coll. Cardiol. 2010, 55, 2816–2821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curzen, N.; Rana, O.; Nicholas, Z.; Golledge, P.; Zaman, A.; Oldroyd, K.; Hanratty, C.; Banning, A.; Wheatcroft, S.; Hobson, A.; et al. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain? The RIPCORD study. Circ. Cardiovasc. Interv. 2014, 7, 248–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.-P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.; Head, S.J.; Juni, P.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2014, 35, 2541–2619. [Google Scholar] [PubMed]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Dallas, A.P. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2012, 60, 2564–2603. [Google Scholar]
- Taylor, C.A.; Fonte, T.A.; Min, J.K. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: Scientific basis. J. Am. Coll. Cardiol. 2013, 61, 2233–2241. [Google Scholar] [CrossRef] [Green Version]
- Koo, B.-K.; Erglis, A.; Doh, J.-H.; Daniels, D.V.; Jegere, S.; Kim, H.-S.; Dunning, A.; DeFrance, T.; Lansky, A.; Leipsic, J.; et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noni. J. Am. Coll. Cardiol. 2011, 58, 1989–1997. [Google Scholar] [CrossRef] [Green Version]
- Min, J.K.; Leipsic, J.; Pencina, M.J.; Berman, D.S.; Koo, B.-K.; van Mieghem, C.; Erglis, A.; Lin, F.Y.; Dunning, A.M.; Apruzzese, P.; et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012, 308, 1237–1245. [Google Scholar] [CrossRef]
- Nørgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: The NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J. Am. Coll. Cardiol. 2014, 63, 1145–1155. [Google Scholar] [CrossRef] [Green Version]
- Douglas, P.S.; Pontone, G.; Hlatky, M.A.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: The prospective longitudinal trial of FFR(CT): Outcome and resource impacts study. Eur. Heart J. 2015, 36, 3359–3367. [Google Scholar]
- Douglas, P.S.; De Bruyne, B.; Pontone, G.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. 1-Year Outcomes of FFRCT-Guided Care in Patients With Suspected Coronary Disease: The PLATFORM Study. J. Am. Coll. Cardiol. 2016, 68, 435–445. [Google Scholar] [CrossRef]
- Hlatky, M.A.; De Bruyne, B.; Pontone, G.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. Quality-of-Life and Economic Outcomes of Assessing Fractional Flow Reserve With Computed Tomography Angiography: PLATFORM. J. Am. Coll. Cardiol. 2015, 66, 2315–2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbara, S.; Blanke, P.; Maroules, C.D.; Cheezum, M.; Choi, A.D.; Han, B.K.; Marwan, M.; Naoum, C.; Norgaard, B.L.; Rubinshtein, R.; et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2016, 10, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Leipsic, J.; Abbara, S.; Achenbach, S.; Cury, R.; Earls, J.P.; Mancini, G.J.; Nieman, K.; Pontone, G.; Raff, G.L. SCCT guidelines for the interpretation and reporting of coronary CT angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J. Cardiovasc. Comput. Tomogr. 2014, 8, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Rabbat, M.G.; Berman, D.S.; Kern, M.; Raff, G.; Chinnaiyan, K.; Koweek, L.; Shaw, L.J.; Blanke, P.; Scherer, M.; Jensen, J.M.; et al. Interpreting results of coronary computed tomography angiography-derived fractional flow reserve in clinical practice. J. Cardiovasc. Comput. Tomogr. 2017, 11, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Kueh, S.H.; Mooney, J.; Ohana, M.; Kim, U.; Blanke, P.; Grover, R.; Sellers, S.; Ellis, J.; Murphy, D.; Haque, C.; et al. Fractional flow reserve derived from coronary computed tomography angiography reclassification rate using value distal to lesion compared to lowest value. J. Cardiovasc. Comput. Tomogr. 2017, 11, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, K.M.; Akasaka, T.; Amano, T.; Bax, J.J.; Blanke, P.; De Bruyne, B.; Kawasaki, T.; Leipsic, J.; Matsuo, H.; Morino, Y.; et al. Rationale, design and goals of the HeartFlow assessing diagnostic value of non-invasive FFRCT in Coronary Care (ADVANCE) registry. J. Cardiovasc. Comput. Tomogr. 2017, 11, 62–67. [Google Scholar] [CrossRef]
- Ball, C.; Pontone, G.; Rabbat, M. Fractional Flow Reserve Derived from Coronary Computed Tomography Angiography Datasets: The Next Frontier in Noninvasive Assessment of Coronary Artery Disease. Biomed. Res. Int. 2018, 11, 462–467. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J. Am. Coll. Cardiol. 2012, 79, 453–495. [Google Scholar]
- Williams, M.C.; Hunter, A.; Shah, A.S.V.; Assi, V.; Lewis, S.; Smith, J.; Berry, C.; Boon, N.A.; Clark, E.; Flather, M.; et al. Use of Coronary Computed Tomographic Angiography to Guide Management of Patients With Coronary Disease. J. Am. Coll. Cardiol. 2016, 67, 1759–1768. [Google Scholar] [CrossRef] [Green Version]
- Newby, D.E.; Adamson, P.D.; Berry, C.; Boon, N.A.; Dweck, M.R.; Flather, M.; Forbes, J.; Hunter, A.; Lewis, S.; MacLean, S.; et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar]
- Foy, A.J.; Dhruva, S.S.; Peterson, B.; Mandrola, J.M.; Morgan, D.J.; Redberg, R.F. Coronary Computed Tomography Angiography vs Functional Stress Testing for Patients with Suspected Coronary Artery Disease: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2017, 177, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Boden, W.E.; O’Rourke, R.A.; Teo, K.K.; Hartigan, P.M.; Maron, D.J.; Kostuk, W.J.; Knudtson, M.; Dada, M.; Casperson, P.; Harris, C.L.; et al. Optimal medical therapy with or without PCI for stable coronary disease. N. Engl. J. Med. 2007, 356, 1503–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BARI 2D Study Group; Frye, R.L.; August, P.; Brooks, M.M.; Hardison, R.M.; Kelsey, S.F.; MacGregor, J.M.; Orchard, T.J.; Chaitman, B.R.; Genuth, S.M.; et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N. Engl. J. Med. 2009, 360, 2503–2515. [Google Scholar]
- Al-Lamee, R.; Thompson, D.; Dehbi, H.-M.; Sen, S.; Tang, K.; Davies, J.; Keeble, T.; Mielewczik, M.; Kaprielian, R.; Malik, I.S.; et al. Percutaneous coronary intervention in stable angina (ORBITA): A double-blind, randomised controlled trial. Lancet 2018, 391, 31–41. [Google Scholar] [CrossRef]
- Zimmermann, F.M.; Ferrara, A.; Johnson, N.P.; van Nunen, L.X.; Escaned, J.; Albertsson, P.; Erbel, R.; Legrand, V.; Gwon, H.; Remkes, W.; et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur. Heart J. 2015, 36, 3182–3188. [Google Scholar] [CrossRef] [Green Version]
- Tonino, P.A.L.; De Bruyne, B.; Pijls, N.H.J.; Siebert, U.; Ikeno, F.; Veer, M.V.; Klauss, V.; Manoharan, G.; Engstrom, T.; Oldroyd, K.G.; et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef] [Green Version]
- De Bruyne, B.; Pijls, N.H.J.; Kalesan, B.; Barbato, E.; Tonino, P.A.L.; Piroth, Z.; Jagic, N.; Mobius-Winkler, S.; Rioufol, G.; Witt, N.; et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N. Engl. J. Med. 2012, 367, 991–1001. [Google Scholar] [CrossRef] [Green Version]
- Fearon, W.F.; Nishi, T.; De Bruyne, B.; Boothroyd, D.B.; Barbato, E.; Tonino, P.; Juni, P.; Pijls, N.; Hlatky, M. Clinical Outcomes and Cost-Effectiveness of Fractional Flow Reserve-Guided Percutaneous Coronary Intervention in Patients With Stable Coronary Artery Disease: Three-Year Follow-Up of the FAME 2 Trial (Fractional Flow Reserve Versus Angiography for Multive. Circulation 2017, 137, 480–487. [Google Scholar] [CrossRef]
- Timmis, A.; Roobottom, C.A. National Institute for Health and Care Excellence updates the stable chest pain guideline with radical changes to the diagnostic paradigm. Heart 2017, 103, 982–986. [Google Scholar] [CrossRef]
- Coronary Computed Tomography Angiography with Selective Noninvasive Fractional Flow Reserve. 2017. Available online: https://app.evidencestreet.com (accessed on 20 February 2020).
- Nørgaard, B.L.; Hjort, J.; Gaur, S.; Hansson, N.; Bøtker, H.E.; Leipsic, J.; Mathiassen, O.N.; Grove, E.L.; Pedersen, K.; Christiansen, E.H.; et al. Clinical Use of Coronary CTA-Derived FFR for Decision-Making in Stable CAD. JACC Cardiovasc. Imaging 2017, 10, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Nørgaard, B.L.; Gormsen, L.C.; Bøtker, H.E.; Partner, E.; Nielsen, L.H.; Mathiassen, O.N.; Grove, E.L.; Ovrehus, K.A.; Gaur, S.; Leipsic, J.; et al. Myocardial Perfusion Imaging Versus Computed Tomography Angiography-Derived Fractional Flow Reserve Testing in Stable Patients With Intermediate-Range Coronary Lesions: Influence on Downstream Diagnostic Workflows and Invasive Angiography Findings. J. Am. Heart Assoc. 2017, 6, e005587. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.M.; Bøtker, H.E.; Mathiassen, O.N.; Grove, E.L.; Øvrehus, K.A.; Pedersen, K.B.; Terkelsen, C.J.; Christiansen, E.H.; Maeng, M.; Leipsic, J.; et al. Computed tomography derived fractional flow reserve testing in stable patients with typical angina pectoris: Influence on downstream rate of invasive coronary angiography. Eur. Heart J. Cardiovasc. Imaging 2017, 19, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Leipsic, J.; Yang, T.-H.; Thompson, A.; Koo, B.-K.; Mancini, G.B.J.; Taylor, C.; Budoff, M.J.; Park, H.B.; Berman, D.S.; Min, J.K. CT angiography (CTA) and diagnostic performance of noninvasive fractional flow reserve: Results from the Determination of Fractional Flow Reserve by Anatomic CTA (DeFACTO) study. Am. J. Roentgenol. 2014, 202, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Pijls, N.H.J.; Sels, J.-W.E.M. Functional Measurement of Coronary Stenosis. J. Am. Coll. Cardiol. 2012, 59, 1045–1057. [Google Scholar] [CrossRef] [Green Version]
- Feuchtner, G.M.; Barbieri, F.; Langer, C.; Beyer, C.; Widmann, G.; Friedrich, G.J.; Cartes-Zumelzu, F.; Plank, F. Non obstructive high-risk plaque but not calcified by coronary CTA, and the G-score predict ischemia. J. Cardiovasc. Comput. Tomogr. 2019, 13, 305–314. [Google Scholar] [CrossRef]
- Bradley, S.M.; Maddox, T.M.; Stanislawski, M.A.; O’Donnell, C.I.; Grunwald, G.K.; Tsai, T.T.; Ho, P.M.; Peterson, E.D.; Rumsfeld, J.S. Normal coronary rates for elective angiography in the Veterans Affairs Healthcare System: Insights from the VA CART program (veterans affairs clinical assessment reporting and tracking). J. Am. Coll. Cardiol. 2014, 63, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Hermann, L.K.; Newman, D.H.; Pleasant, W.A.; Rojanasarntikul, D.; Lakoff, D.; Goldberg, S.A.; Duvall, W.L.; Henzlova, M.J. Yield of routine provocative cardiac testing among patients in an emergency department-based chest pain unit. JAMA Intern. Med. 2013, 173, 1128–1133. [Google Scholar] [CrossRef] [Green Version]
- Hwang, I.-C.; Kim, Y.-J.; Kim, K.-H.; Shin, D.-H.; Lee, S.-P.; Kim, H.-K.; Sohn, D.W. Diagnostic yield of coronary angiography in patients with acute chest pain: Role of noninvasive test. Am. J. Emerg. Med. 2014, 32, 1–6. [Google Scholar] [CrossRef]
- Ko, D.T.; Tu, J.V.; Austin, P.C.; Wijeysundera, H.C.; Samadashvili, Z.; Guo, H.; Cantor, W.J.; Hannan, E.L. Prevalence and extent of obstructive coronary artery disease among patients undergoing elective coronary catheterization in New York State and Ontario. JAMA 2013, 310, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Roifman, I.; Wijeysundera, H.C.; Austin, P.C.; Rezai, M.R.; Wright, G.A.; Tu, J.V. Comparison of Anatomic and Clinical Outcomes in Patients Undergoing Alternative Initial Noninvasive Testing Strategies for the Diagnosis of Stable Coronary Artery Disease. J. Am. Heart Assoc. 2017, 6, e005462. [Google Scholar] [CrossRef]
- Wijeysundera, H.C.; Qiu, F.; Bennell, M.C.; Natarajan, M.K.; Cantor, W.J.; Smith, S.; Kingsbury, K.J.; Ko, D.T. Impact of system and physician factors on the detection of obstructive coronary disease with diagnostic angiography in stable ischemic heart disease. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 648–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Characteristic | Coronary CTA + FFRCT (n = 387) | Coronary CTA (n = 44) |
|---|---|---|
| Age | 58.9 (13.1) | 59 (10) |
| BMI (kg/m2) | 29.7 (6.0) | 28.9 (7.6) |
| Male | 190 (49.2) | 18 (41.0) |
| Diabetes Mellitus | 63 (16.5) | 8 (18.2) |
| Hyperlipidemia | 244 (64.0) | 24 (54.5) |
| Hypertension | 229 (60.1) | 24 (54.5) |
| Smoker | ||
| Current | 42 (11.1) | 8 (18.2) |
| Ex | 128 (33.8) | 7 (15) |
| Never | 209 (55.2) | 29 (66.8) |
| Anginal Typicality | ||
| Asymptomatic | 52 (13.7) | 4 (9) |
| Atypical | 189 (49.7) | 32 (72.7) |
| Non-anginal | 102 (26.8) | 1 (2.3) |
| Typical | 37 (9.7) | 7 (16) |
| Prior Functional Stress Test | 149 (38.5) | 29 (65.9) |
| Diamond Forrester Score | ||
| Low | 13 (5.1) | 6 (13.6) |
| Intermediate | 228 (90.1) | 37 (84.1) |
| High | 12 (4.7) | 1 (2.3) |
| Pre-CTA Aspirin | 125 (32.8) | 13 (29.5) |
| Pre-CTA Statin | 188 (49.3) | 18 (41) |
| Pre-CTA Beta-blocker | 95 (24.9) | 13 (29.5) |
| Pre-CTA Calcium channel blocker | 60 (15.8) | 8 (18.2) |
| Pre-CTA ACEi | 78 (20.5) | 4 (9.1) |
| Pre-CTA ARB | 63 (16.5) | 5 (11.4) |
| Pre-CTA Thiazide | 80 (21) | 7 (15.9) |
| Pre-CTA Nitrate | 2 (0.5) | 1 (2.3) |
| Acquisition Characteristic | Coronary CTA |
|---|---|
| Heart Rate, bpm; Mean ± SD (Range) | 59 ± 7 (40–80) |
| Pre-scan administration of nitrates | 376 (99.7%) |
| Pre-scan administration of beta-blockers | 269 (71.2%) |
| Prospective acquisition | 42 (10.9%) |
| Retrospective acquisition | 345 (89.1%) |
| Effective CTA radiation dose, mSv | |
| Prospective acquisition | 4.8 ± 1.8 |
| Retrospective acquisition | 10.9 ± 6.0 |
| FFRCT | Stenosis | n | ICA (%) | PCI (%) | CABG (%) | Revascularization (%) |
|---|---|---|---|---|---|---|
| Not available | ≥50% | 14 | 11 (79) | 5 (36) | 1 (7) | 6 (43) |
| <50% | 14 | 3 (21) | 1 (7) | 1 (7) | 2 (14) | |
| ≤0.80 | ≥50% | 67 | 41 (61) | 18 (27) | 9 (13) | 27 (40) |
| <50% | 59 | 5 (9) | 3 (5) | 0 (0) | 3 (5) | |
| >0.80 | ≥50% | 40 | 3 (8) | 1 (3) | 0 (0) | 1 (3) |
| <50% | 190 | 2 (1) | 0 (0) | (0) | (0) | |
| Total | 384 | 65 (17) | 28 (7) | 11 (3) | 39 (10) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabbat, M.; Leipsic, J.; Bax, J.; Kauh, B.; Verma, R.; Doukas, D.; Allen, S.; Pontone, G.; Wilber, D.; Mathew, V.; et al. Fractional Flow Reserve Derived from Coronary Computed Tomography Angiography Safely Defers Invasive Coronary Angiography in Patients with Stable Coronary Artery Disease. J. Clin. Med. 2020, 9, 604. https://doi.org/10.3390/jcm9020604
Rabbat M, Leipsic J, Bax J, Kauh B, Verma R, Doukas D, Allen S, Pontone G, Wilber D, Mathew V, et al. Fractional Flow Reserve Derived from Coronary Computed Tomography Angiography Safely Defers Invasive Coronary Angiography in Patients with Stable Coronary Artery Disease. Journal of Clinical Medicine. 2020; 9(2):604. https://doi.org/10.3390/jcm9020604
Chicago/Turabian StyleRabbat, Mark, Jonathon Leipsic, Jeroen Bax, Brian Kauh, Rina Verma, Demetrios Doukas, Sorcha Allen, Gianluca Pontone, David Wilber, Verghese Mathew, and et al. 2020. "Fractional Flow Reserve Derived from Coronary Computed Tomography Angiography Safely Defers Invasive Coronary Angiography in Patients with Stable Coronary Artery Disease" Journal of Clinical Medicine 9, no. 2: 604. https://doi.org/10.3390/jcm9020604
APA StyleRabbat, M., Leipsic, J., Bax, J., Kauh, B., Verma, R., Doukas, D., Allen, S., Pontone, G., Wilber, D., Mathew, V., Rogers, C., & Lopez, J. (2020). Fractional Flow Reserve Derived from Coronary Computed Tomography Angiography Safely Defers Invasive Coronary Angiography in Patients with Stable Coronary Artery Disease. Journal of Clinical Medicine, 9(2), 604. https://doi.org/10.3390/jcm9020604