Dermatopontin, A Novel Adipokine Promoting Adipose Tissue Extracellular Matrix Remodelling and Inflammation in Obesity †
Abstract
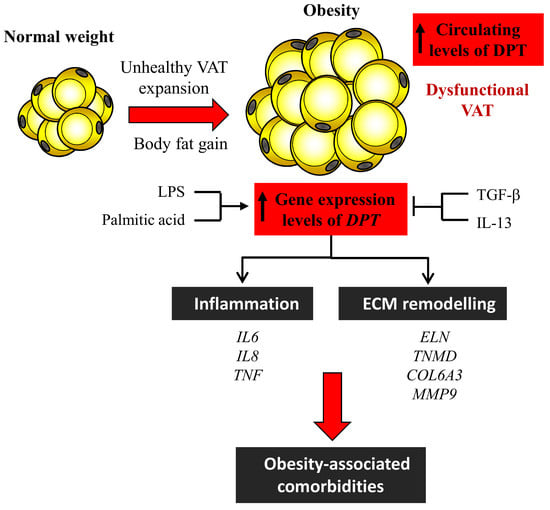
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Analytical Measurements
2.3. RNA Isolation and Real-Time PCR
2.4. Human Adipocyte Culture and Treatment
2.5. Statistical Analysis
3. Results
3.1. Circulating Levels of DPT are Increased in Obesity
3.2. Obesity and Obesity-Associated T2D Increase Gene Expression Levels of DPT in VAT
3.3. Effect of Inflammatory and Anti-Inflammatory Factors in DPT Expression in Visceral Adipocytes
3.4. Dermatopontin Upregulates ECM Remodelling and Inflammation-Related Genes in Visceral Adipocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Invest. 2017, 127, 74–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Tordjman, J.; Clement, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Ambrosi, J.; Catalán, V.; Diez-Caballero, A.; Martínez-Cruz, L.A.; Gil, M.J.; García-Foncillas, J.; Cienfuegos, J.A.; Salvador, J.; Mato, J.M.; Frühbeck, G. Gene expression profile of omental adipose tissue in human obesity. FASEB J. 2004, 18, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Okamoto, O.; Ishikawa, K.; Sumiyoshi, H.; Matsuo, N.; Yoshioka, H.; Nomizu, M.; Shimada, T.; Fujiwara, S. Dermatopontin interacts with fibronectin, promotes fibronectin fibril formation, and enhances cell adhesion. J. Biol. Chem. 2011, 286, 14861–14869. [Google Scholar] [CrossRef] [Green Version]
- Kato, A.; Okamoto, O.; Wu, W.; Matsuo, N.; Kumai, J.; Yamada, Y.; Katagiri, F.; Nomizu, M.; Fujiwara, S. Identification of fibronectin binding sites in dermatopontin and their biological function. J. Dermatol. Sci. 2014, 76, 51–59. [Google Scholar] [CrossRef]
- Wu, W.; Okamoto, O.; Kato, A.; Matsuo, N.; Kumai, J.; Nomizu, M.; Fujiwara, S. Functional peptide of dermatopontin produces fibrinogen fibrils and modifies its biological activity. J. Dermatol. Sci. 2014, 76, 34–43. [Google Scholar] [CrossRef]
- MacBeath, J.R.; Shackleton, D.R.; Hulmes, D.J. Tyrosine-rich acidic matrix protein (TRAMP) accelerates collagen fibril formation in vitro. J. Biol. Chem. 1993, 268, 19826–19832. [Google Scholar]
- Okamoto, O.; Fujiwara, S. Dermatopontin, a novel player in the biology of the extracellular matrix. Connect. Tissue Res. 2006, 47, 177–189. [Google Scholar] [CrossRef]
- Okamoto, O.; Suzuki, Y.; Kimura, S.; Shinkai, H. Extracellular matrix 22-kDa protein interacts with decorin core protein and is expressed in cutaneous fibrosis. J. Biochem. 1996, 119, 106–114. [Google Scholar] [CrossRef]
- Lefebvre, P.; Lalloyer, F.; Bauge, E.; Pawlak, M.; Gheeraert, C.; Dehondt, H.; Vanhoutte, J.; Woitrain, E.; Hennuyer, N.; Mazuy, C.; et al. Interspecies NASH disease activity whole-genome profiling identifies a fibrogenic role of PPARa-regulated dermatopontin. JCI Insight 2017, 2, e92264. [Google Scholar] [CrossRef]
- Takeda, U.; Utani, A.; Wu, J.; Adachi, E.; Koseki, H.; Taniguchi, M.; Matsumoto, T.; Ohashi, T.; Sato, M.; Shinkai, H. Targeted disruption of dermatopontin causes abnormal collagen fibrillogenesis. J. Invest. Dermatol. 2002, 119, 678–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, L.J.; Bentley, A.J.; Nieduszynski, I.A.; Talabani, S.; Thomson, A.; Utani, A.; Shinkai, H.; Fullwood, N.J.; Brown, G.M. The role of dermatopontin in the stromal organization of the cornea. Invest. Ophthalmol. Vis. Sci. 2006, 47, 3303–3310. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic dysregulation and adipose tissue fibrosis: Role of collagen VI. Mol. Cell. Biol. 2009, 29, 1575–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Invest. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [Green Version]
- Krishnaswamy, V.R.; Manikandan, M.; Munirajan, A.K.; Vijayaraghavan, D.; Korrapati, P.S. Expression and integrity of dermatopontin in chronic cutaneous wounds: A crucial factor in impaired wound healing. Cell Tissue Res. 2014, 358, 833–841. [Google Scholar] [CrossRef]
- Okamoto, O.; Fujiwara, S.; Abe, M.; Sato, Y. Dermatopontin interacts with transforming growth factor b and enhances its biological activity. Biochem. J. 1999, 337, 537–541. [Google Scholar] [CrossRef]
- Liu, X.; Meng, L.; Shi, Q.; Liu, S.; Cui, C.; Hu, S.; Wei, Y. Dermatopontin promotes adhesion, spreading and migration of cardiac fibroblasts in vitro. Matrix Biol. 2013, 32, 23–31. [Google Scholar] [CrossRef]
- Okamoto, O.; Hozumi, K.; Katagiri, F.; Takahashi, N.; Sumiyoshi, H.; Matsuo, N.; Yoshioka, H.; Nomizu, M.; Fujiwara, S. Dermatopontin promotes epidermal keratinocyte adhesion via a3b1 integrin and a proteoglycan receptor. Biochemistry 2010, 49, 147–155. [Google Scholar] [CrossRef]
- Kim, T.; Ahmad, K.; Shaikh, S.; Jan, A.T.; Seo, M.G.; Lee, E.J.; Choi, I. Dermatopontin in skeletal muscle extracellular matrix regulates myogenesis. Cells 2019, 8, 332. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef] [Green Version]
- Kleiner, D.E.; Brunt, E.M. Nonalcoholic fatty liver disease: Pathologic patterns and biopsy evaluation in clinical research. Semin. Liver Dis. 2012, 32, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Ambrosi, J.; Catalán, V.; Rodríguez, A.; Andrada, P.; Ramírez, B.; Ibañez, P.; Vila, N.; Romero, S.; Margall, M.A.; Gil, M.J.; et al. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care 2014, 37, 2813–2821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Ambrosi, J.; Rodríguez, A.; Catalán, V.; Ramírez, B.; Silva, C.; Rotellar, F.; Gil, M.J.; Salvador, J.; Frühbeck, G. Serum retinol-binding protein 4 is not increased in obesity or obesity-associated type 2 diabetes mellitus, but is reduced after relevant reductions in body fat following gastric bypass. Clin. Endocrinol. 2008, 69, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Keach, J.C.; Batts, K.P.; Lindor, K.D. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999, 30, 1356–1362. [Google Scholar] [CrossRef]
- Muruzabal, F.J.; Frühbeck, G.; Gómez-Ambrosi, J.; Archanco, M.; Burrell, M.A. Immunocytochemical detection of leptin in non-mammalian vertebrate stomach. Gen. Comp. Endocrinol. 2002, 128, 149–152. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Valentí, V.; Moncada, R.; Landecho, M.F.; Silva, C.; Salvador, J.; Frühbeck, G. Increased interleukin-32 levels in obesity promote adipose tissue inflammation and extracellular matrix remodeling: Effect of weight loss. Diabetes 2016, 65, 3636–3648. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, A.; Gómez-Ambrosi, J.; Catalán, V.; Gil, M.J.; Becerril, S.; Sainz, N.; Silva, C.; Salvador, J.; Colina, I.; Frühbeck, G. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int. J. Obes. 2009, 33, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, K.; Okamoto, O.; Shinkai, H. Dermatopontin expression is decreased in hypertrophic scar and systemic sclerosis skin fibroblasts and is regulated by transforming growth factor-b1, interleukin-4, and matrix collagen. J. Invest. Dermatol. 1999, 112, 706–710. [Google Scholar] [CrossRef] [Green Version]
- Cansancao, K.; Silva Monteiro, L.; Carvalho Leite, N.; Davalos, A.; Tavares do Carmo, M.D.G.; Arantes Ferreira Peres, W. Advanced liver fibrosis is independently associated with palmitic acid and insulin levels in patients with non-alcoholic fatty liver disease. Nutrients 2018, 10, 1586. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, Y.; Imajo, K.; Honda, Y.; Kessoku, T.; Tomeno, W.; Kato, S.; Fujita, K.; Yoneda, M.; Saito, S.; Saigusa, Y.; et al. Palmitate-induced lipotoxicity is crucial for the pathogenesis of nonalcoholic fatty liver disease in cooperation with gut-derived endotoxin. Sci. Rep. 2018, 8, 11365. [Google Scholar] [CrossRef] [Green Version]
- Lin Chun, T.H.; Kang, L. Adipose extracellular matrix remodelling in obesity and insulin resistance. Biochem. Pharmacol. 2016, 119, 8–16. [Google Scholar]
- Divoux, A.; Clément, K. Architecture and the extracellular matrix: The still unappreciated components of the adipose tissue. Obes. Rev. 2011, 12, e494–e503. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Minetti, C.A.; Nakhasi, H.L.; Chen, S.W.; Barbehenn, E.; Nunes, P.H.; Nguyen, N.Y. Isolation, cDNA cloning, and characterization of an 18-kDa hemagglutinin and amebocyte aggregation factor from Limulus polyphemus. J. Biol. Chem. 1992, 267, 22452–22459. [Google Scholar] [PubMed]
- Datta, R.; Podolsky, M.J.; Atabai, K. Fat fibrosis: Friend or foe? JCI Insight 2018, 3, 122289. [Google Scholar] [CrossRef]
- Le Dour, C.; Wu, W.; Bereziat, V.; Capeau, J.; Vigouroux, C.; Worman, H.J. Extracellular matrix remodeling and transforming growth factor-b signaling abnormalities induced by lamin A/C variants that cause lipodystrophy. J. Lipid Res. 2017, 58, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Pasarica, M.; Gowronska-Kozak, B.; Burk, D.; Remedios, I.; Hymel, D.; Gimble, J.; Ravussin, E.; Bray, G.A.; Smith, S.R. Adipose tissue collagen VI in obesity. J. Clin. Endocrinol. Metab. 2009, 94, 5155–5162. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Park, J.; Gupta, O.T.; Holland, W.L.; Auerbach, P.; Zhang, N.; Goncalves Marangoni, R.; Nicoloro, S.M.; Czech, M.P.; Varga, J.; et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Blaise, S.; Romier, B.; Kawecki, C.; Ghirardi, M.; Rabenoelina, F.; Baud, S.; Duca, L.; Maurice, P.; Heinz, A.; Schmelzer, C.E.; et al. Elastin-derived peptides are new regulators of insulin resistance development in mice. Diabetes 2013, 62, 3807–3816. [Google Scholar] [CrossRef]
- Martínez-Santibañez, G.; Singer, K.; Cho, K.W.; Del Proposto, J.L.; Mergian, T.; Lumeng, C.N. Obesity-induced remodeling of the adipose tissue elastin network is independent of the metalloelastase MMP-12. Adipocyte 2015, 4, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Bouloumié, A.; Sengenes, C.; Portolan, G.; Galitzky, J.; Lafontan, M. Adipocyte produces matrix metalloproteinases 2 and 9: Involvement in adipose differentiation. Diabetes 2001, 50, 2080–2086. [Google Scholar] [CrossRef] [Green Version]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Silva, C.; Rotellar, F.; Gil, M.J.; Cienfuegos, J.A.; Salvador, J.; Frühbeck, G. Increased adipose tissue expression of lipocalin-2 in obesity is related to inflammation and matrix metalloproteinase-2 and metalloproteinase-9 activities in humans. J. Mol. Med. 2009, 87, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Giannandrea, M.; Parks, W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Model. Mech. 2014, 7, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancha, A.; Rodríguez, A.; Catalán, V.; Becerril, S.; Sainz, N.; Ramírez, B.; Burrell, M.A.; Salvador, J.; Frühbeck, G.; Gómez-Ambrosi, J. Osteopontin deletion prevents the development of obesity and hepatic steatosis via impaired adipose tissue matrix remodeling and reduced inflammation and fibrosis in adipose tissue and liver in mice. PLoS ONE 2014, 9, e98398. [Google Scholar] [CrossRef] [PubMed]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saiki, A.; Olsson, M.; Jernas, M.; Gummesson, A.; McTernan, P.G.; Andersson, J.; Jacobson, P.; Sjoholm, K.; Olsson, B.; Yamamura, S.; et al. Tenomodulin is highly expressed in adipose tissue, increased in obesity, and down-regulated during diet-induced weight loss. J. Clin. Endocrinol. Metab. 2009, 94, 3987–3994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Docheva, D.; Hunziker, E.B.; Fassler, R.; Brandau, O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol. Cell. Biol. 2005, 25, 699–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senol-Cosar, O.; Flach, R.J.; DiStefano, M.; Chawla, A.; Nicoloro, S.; Straubhaar, J.; Hardy, O.T.; Noh, H.L.; Kim, J.K.; Wabitsch, M.; et al. Tenomodulin promotes human adipocyte differentiation and beneficial visceral adipose tissue expansion. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Krishnaswamy, V.R.; Balaguru, U.M.; Chatterjee, S.; Korrapati, P.S. Dermatopontin augments angiogenesis and modulates the expression of transforming growth factor b1 and integrin a3 b1 in endothelial cells. Eur. J. Cell Biol. 2017, 96, 266–275. [Google Scholar] [CrossRef]
- Seifert, L.; Deutsch, M.; Alothman, S.; Alqunaibit, D.; Werba, G.; Pansari, M.; Pergamo, M.; Ochi, A.; Torres-Hernandez, A.; Levie, E.; et al. Dectin-1 regulates hepatic fibrosis and hepatocarcinogenesis by suppressing TLR4 signaling pathways. Cell Rep. 2015, 13, 1909–1921. [Google Scholar] [CrossRef] [Green Version]



| Lean | Obese NG | Obese T2D | |
|---|---|---|---|
| n (males, females) | 13 (5, 8) | 21 (6, 15) | 20 (8, 12) |
| Age (years) | 38 ± 2 | 41 ± 3 | 42 ± 3 |
| BMI (kg/m2) | 21.8 ± 0.9 | 41.6 ± 1.3 ** | 46.5 ± 1.3 ***,†† |
| Body fat (%) | 22.6 ± 2.1 | 53.2 ± 1.4 *** | 52.1 ± 2.1 *** |
| Waist circumference (cm) | 75 ± 3 | 119 ± 3 *** | 129 ± 2 ***,† |
| Hip circumference (cm) | 94 ± 1 | 127 ± 2 *** | 136 ± 3 ***,† |
| Waist-to-hip ratio | 0.79 ± 0.02 | 0.94 ± 0.02 *** | 0.96 ± 0.02 *** |
| Neck circumference (cm) | 34 ± 1 | 38 ± 1 ** | 42 ± 1 **,†† |
| Fasting glucose (mg/dL) | 85 ± 6 | 86 ± 2 | 123 ± 6 **,††† |
| 2h OGTT glucose (mg/dL) | – | 112 ± 3 | 196 ± 14 ††† |
| Fasting insulin (µU/mL) | 7.2 ± 1.3 | 17.1 ± 1.4 | 22.2 ± 3.8 |
| 2h OGTT insulin (µU/mL) | – | 72.7 ± 8.6 | 140.0 ± 18.1 ††† |
| HOMA | 1.6 ± 0.4 | 4.0 ± 1.1 | 6.3 ± 1.3 |
| QUICKI | 0.369 ± 0.016 | 0.334 ± 0.010 | 0.305 ± 0.007 ** |
| Triglycerides (mg/dL) | 67 ± 12 | 97 ± 7 | 143 ± 13 **,†† |
| Cholesterol (mg/dL) | 171 ± 8 | 195 ± 10 | 189 ± 7 |
| LDL-cholesterol (mg/dL) | 88 ± 5 | 119 ± 7 | 113 ± 6 |
| HDL-cholesterol (mg/dL) | 69 ± | 56 ± 5 | 47 ± 3 * |
| Leptin (ng/mL) | 8.9 ± 2.4 | 58.2 ± 5.9 *** | 40.8 ± 8.4 ** |
| CRP (mg/L) | 0.53 ± 0.08 | 9.0 ± 2.1 *** | 8.1 ± 1.8 *** |
| Fibrinogen (mg/dL) | 194 ± 12 | 402 ± 18 *** | 354 ± 23 *** |
| von Willebrand factor (%) | 57 ± 10 | 143 ± 15 ** | 131 ± 14 ** |
| Homocysteine (µmol/L) | 6.6 ± 0.5 | 9.4 ± 0.8 | 10.4 ± 0.6 ** |
| AST (U/L) | 12 ± 1 | 18 ± 4 | 16 ± 4 |
| ALT (U/L) | 6 ± 3 | 22 ± 3 | 28 ± 2 ** |
| AST/ALT | 2.24 ± 0.14 | 0.87 ± 0.08 *** | 0.67 ± 0.06 *** |
| ALP (U/L) | 74 ± 6 | 90 ± 6 | 92 ± 7 |
| γ-GT (U/L) | 13 ± 3 | 19 ± 3 | 30 ± 5 |
| Circulating Levels of DPT | ||
|---|---|---|
| r | p | |
| Age | 0.17 | 0.224 |
| BMI | 0.44 | <0.001 |
| BF | 0.35 | 0.009 |
| Waist circumference | 0.53 | <0.001 |
| Hip circumference | 0.36 | 0.008 |
| Waist-to-hip ratio | 0.53 | <0.001 |
| Neck circumference | 0.49 | <0.001 |
| Fasting glucose | 0.01 | 0.934 |
| Fasting insulin | 0.43 | 0.005 |
| HOMA | 0.34 | 0.032 |
| QUICKI | –0.26 | 0.106 |
| Triglycerides | 0.31 | 0.052 |
| Cholesterol | 0.18 | 0.262 |
| LDL-cholesterol | 0.05 | 0.741 |
| HDL-cholesterol | –0.49 | <0.001 |
| Leptin | 0.27 | 0.122 |
| CRP | 0.06 | 0.706 |
| Fibrinogen | 0.01 | 0.933 |
| von Willebrand factor | 0.16 | 0.357 |
| Homocystein | 0.46 | 0.006 |
| AST | 0.09 | 0.555 |
| ALT | 0.02 | 0.907 |
| AST/ALT | –0.36 | 0.019 |
| ALP | 0.09 | 0.549 |
| γ-GT | 0.02 | 0.882 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unamuno, X.; Gómez-Ambrosi, J.; Ramírez, B.; Rodríguez, A.; Becerril, S.; Valentí, V.; Moncada, R.; Silva, C.; Salvador, J.; Frühbeck, G.; et al. Dermatopontin, A Novel Adipokine Promoting Adipose Tissue Extracellular Matrix Remodelling and Inflammation in Obesity. J. Clin. Med. 2020, 9, 1069. https://doi.org/10.3390/jcm9041069
Unamuno X, Gómez-Ambrosi J, Ramírez B, Rodríguez A, Becerril S, Valentí V, Moncada R, Silva C, Salvador J, Frühbeck G, et al. Dermatopontin, A Novel Adipokine Promoting Adipose Tissue Extracellular Matrix Remodelling and Inflammation in Obesity. Journal of Clinical Medicine. 2020; 9(4):1069. https://doi.org/10.3390/jcm9041069
Chicago/Turabian StyleUnamuno, Xabier, Javier Gómez-Ambrosi, Beatriz Ramírez, Amaia Rodríguez, Sara Becerril, Víctor Valentí, Rafael Moncada, Camilo Silva, Javier Salvador, Gema Frühbeck, and et al. 2020. "Dermatopontin, A Novel Adipokine Promoting Adipose Tissue Extracellular Matrix Remodelling and Inflammation in Obesity" Journal of Clinical Medicine 9, no. 4: 1069. https://doi.org/10.3390/jcm9041069
APA StyleUnamuno, X., Gómez-Ambrosi, J., Ramírez, B., Rodríguez, A., Becerril, S., Valentí, V., Moncada, R., Silva, C., Salvador, J., Frühbeck, G., & Catalán, V. (2020). Dermatopontin, A Novel Adipokine Promoting Adipose Tissue Extracellular Matrix Remodelling and Inflammation in Obesity. Journal of Clinical Medicine, 9(4), 1069. https://doi.org/10.3390/jcm9041069