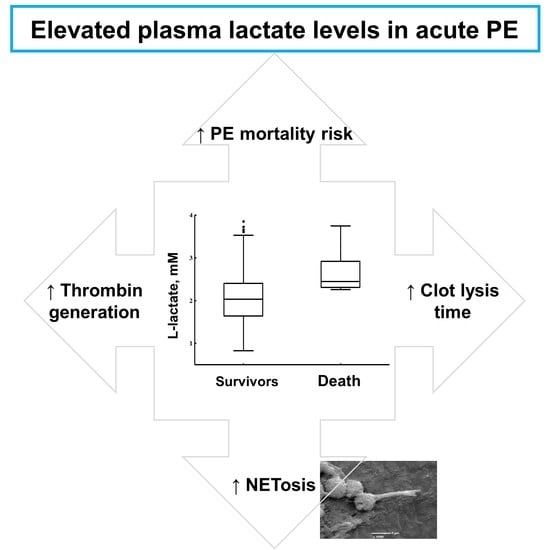
Elevated Lactate Levels in Acute Pulmonary Embolism Are Associated with Prothrombotic Fibrin Clot Properties: Contribution of NETs Formation
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients
2.2. Laboratory Variables
2.3. Fibrin Permeation Analysis
2.4. Plasma Clot Lysis Assay
2.5. Endogenous Thrombin Potential
2.6. Statistics
3. Results
3.1. On Admission
3.2. Follow-up
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.T.; Agnelli, G.; Anderson, F.A.; I Arcelus, J.; Bergqvist, D.; Brecht, J.G.; A Greer, I.; A Heit, J.; Hutchinson, J.L.; Kakkar, A.K.; et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb. Haemost. 2007, 98, 756–764. [Google Scholar] [PubMed]
- Liedl, G.; Nazerian, P.; Pepe, G.; Caviglioli, C.; Grifoni, S.; Vanni, S. Different time course of plasma lactate, troponin I and NT-proBNP concentrations in patients with acute pulmonary embolism. Thromb. Res. 2017, 156, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Respir. J. 2019, 54, 1901647. [Google Scholar]
- Kraut, J.A.; Madias, N.E. Lactic acidosis. N. Engl. J. Med. 2014, 371, 2309–2319. [Google Scholar] [CrossRef]
- Vanni, S.; Viviani, G.; Baioni, M.; Pepe, G.; Nazerian, P.; Socci, F.; Bartolucci, M.; Bartolini, M.; Grifoni, S. Prognostic value of plasma lactate levels among patients with acute pulmonary embolism: The thrombo-embolism lactate outcome study. Ann. Emerg. Med. 2013, 61, 330–338. [Google Scholar] [CrossRef]
- Vanni, S.; Nazerian, P.; Morello, F.; Parisi, M.; Daghini, E.; Pratesi, M.; Bedate, P.; Lobo, J.L.; Jara-Palomares, L.; Portillo, A.K.; et al. Short-term clinical outcome of normotensive patients with acute PE and high plasma lactate. Thorax 2015, 70, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Alarcón, P.; Manosalva, C.; Conejeros, I.; Carretta, M.D.; Muñoz-Caro, T.; Silva, L.M.R.; Taubert, A.; Hermosilla, C.; Hidalgo, M.A.; Burgos, R.A. d(-) Lactic Acid-Induced Adhesion of Bovine Neutrophils onto Endothelial Cells Is Dependent on Neutrophils Extracellular Traps Formation and CD11b Expression. Front. Immunol. 2017, 8, 975. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef] [Green Version]
- Von Brühl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Stakos, D.A.; Kambas, K.; Konstantinidis, T.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Tsironidou, V.; Giatromanolaki, A.; Skendros, P.; Konstantinides, S.; et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur. Heart J. 2015, 36, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Thålin, C.; Hisada, Y.; Lundström, S.; Mackman, N.; Wallén, H. Neutrophil Extracellular Traps: Villains and Targets in Arterial, Venous, and Cancer-Associated Thrombosis. Arter. Thromb. Vasc. Biol. 2019, 39, 1724–1738. [Google Scholar] [CrossRef] [PubMed]
- Longstaff, C.; Varjú, I.; Sótonyi, P.; Szabó, L.; Krumrey, M.; Hoell, A.; Bóta, A.; Varga, Z.; Komorowicz, E.; Kolev, K. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J. Biol. Chem. 2013, 288, 6946–6956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, T.J.; Vu, T.T.; Swystun, L.L.; Dwivedi, D.J.; Mai, S.H.; Weitz, J.I.; Liaw, P.C. Neutrophil extracellular traps promote thrombin generation through plateletdependent and platelet-independent mechanisms. Arter. Thromb. Vasc. Biol. 2014, 34, 1977–1984. [Google Scholar] [CrossRef] [Green Version]
- Ząbczyk, M.; Natorska, J.; Janion-Sadowska, A.; Metzgier-Gumiela, A.; Polak, M.; Plens, K.; Janion, M.; Skonieczny, G.; Mizia-Stec, K.; Undas, A. Prothrombotic fibrin clot properties associated with NETs formation characterize acute pulmonary embolism patients with higher mortality risk. Sci. Rep 2020, in press. [Google Scholar]
- Cieslik, J.; Mrozinska, S.; Broniatowska, E.; Undas, A. Altered plasma clot properties increase the risk of recurrent deep vein thrombosis: A cohort study. Blood 2018, 131, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Pieters, M.; Philippou, H.; Undas, A.; De Lange, Z.; Rijken, D.; Mutch, N.J.; Subcommittee on Factor XIII and Fibrinogen, and the Subcommittee on Fibrinolysis. An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. Subcell. Biochem. 2017, 82, 405–456. [Google Scholar]
- Ząbczyk, M.; Stachowicz, A.; Natorska, J.; Olszanecki, R.; Wiśniewski, J.R.; Undas, A. Plasma fibrin clot proteomics in healthy subjects: Relation to clot permeability and lysis time. J. Proteom. 2019, 208, 103487. [Google Scholar] [CrossRef]
- Stachowicz, A.; Zabczyk, M.; Natorska, J.; Suski, M.; Olszanecki, R.; Korbut, R.; Wiśniewski, J.R.; Undas, A. Differences in plasma fibrin clot composition in patients with thrombotic antiphospholipid syndrome compared with venous thromboembolism. Sci. Rep. 2018, 8, 17301. [Google Scholar] [CrossRef]
- Undas, A.; Ariëns, R.A. Fibrin clot structure and function: A role in the pathophysiology of arterial and venous thromboembolic diseases. Arter. Thromb. Vasc. Biol. 2011, 31, e88–e99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heffron, S.P.; Parastatidis, I.; Cuchel, M.; Wolfe, M.L.; Tadesse, M.G.; Mohler, E.R.; Ischiropoulos, H.; Rader, D.J.; Reilly, M. Inflammation induces fibrinogen nitration in experimental human endotoxemia. Free Radic. Biol. Med. 2009, 47, 1140–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meltzer, M.E.; Lisman, T.; de Groot, P.G.; Meijers, J.C.M.; Le Cessie, S.; Doggen, C.J.M.; Rosendaal, F. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood 2010, 116, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, M.; Cuker, A.; Mills, A.; Lightfoot, R.; Fan, Y.; Tang, W.H.W.; Hazen, S.L.; Ischiropoulos, H. Nitrated fibrinogen is a biomarker of oxidative stress in venous thromboembolism. Free Radic. Biol. Med. 2012, 53, 230–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaut, J.P.; Byun, J.; Tran, H.D.; Lauber, W.M.; Carroll, J.A.; Hotchkiss, R.S.; Belaaouaj, A.; Heinecke, J.M. Myeloperoxidase produces nitrating oxidants In Vivo. J. Clin. Investig. 2002, 109, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, D.; Nagarkoti, S.; Sadaf, S.; Chandra, T.; Kumar, S.; Dikshit, M. Glycolysis dependent lactate formation in neutrophils: A metabolic link between NOX-dependent and independent NETosis. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165542. [Google Scholar] [CrossRef]
- Keshari, R.S.; Jyoti, A.; Dubey, M.; Kothari, N.; Kohli, M.; Bogra, J.; Barthwal, M.K.; Dikshit, M. Cytokines induced neutrophil extracellular traps formation: Implication for the inflammatory disease condition. PLoS ONE 2012, 7, e48111. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Kumar, S.; Jyoti, A.; Srinag, B.S.; Keshari, R.S.; Saluja, R.; Verma, A.; Mitra, K.; Barthwal, M.K.; Krishnamurthy, H.; et al. Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide 2010, 22, 226–234. [Google Scholar] [CrossRef]
- Awasthi, D.; Nagarkoti, S.; Kumar, A.; Dubey, M.; Singh, A.K.; Pathak, P.; Chandra, T.; Barthwal, M.K.; Dikshit, M. Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radic. Biol. Med. 2016, 93, 190–203. [Google Scholar] [CrossRef]
- Andersen, L.W.; Mackenhauer, J.; Roberts, J.C.; Berg, K.M.; Cocchi, M.N.; Donnino, M.W. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin. Proc. 2013, 88, 1127–1140. [Google Scholar] [CrossRef] [Green Version]
- Robergs, R.A.; McNulty, C.R.; Minett, G.M.; Holland, J.; Trajano, G. Lactate, not Lactic Acid, is Produced by Cellular Cytosolic Energy Catabolism. Physiology 2018, 33, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, A.S.; Martinod, K.; Seidman, M.A.; Wong, S.L.; Borissoff, J.I.; Piazza, G.; Libby, P.; Goldhaber, S.Z.; Mitchell, R.N.; Wagner, D.D. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J. Thromb. Haemost. 2014, 12, 860–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammollo, C.T.; Semeraro, F.; Xu, J.; Esmon, N.L.; Esmon, C.T. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J. Thromb. Haemost. 2011, 9, 1795–1803. [Google Scholar] [CrossRef]
- Bryk, A.H.; Prior, S.M.; Plens, K.; Konieczynska, M.; Hohendorff, J.; Malecki, M.T.; Butenas, S.; Undas, A. Predictors of neutrophil extracellular traps markers in type 2 diabetes mellitus: Associations with a prothrombotic state and hypofibrinolysis. Cardiovasc. Diabetol. 2019, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Mauracher, L.M.; Posch, F.; Martinod, K.; Grilz, E.; Däullary, T.; Hell, L.; Brostjan, C.; Zielinski, C.; Ay, C.; Wagner, D.D.; et al. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J. Thromb. Haemost. 2018, 16, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Ząbczyk, M.; Królczyk, G.; Czyżewicz, G.; Plens, K.; Prior, S.; Butenas, S.; Undas, A. Altered fibrin clot properties in advanced lung cancer: Strong impact of cigarette smoking. Med. Oncol. 2019, 36, 37. [Google Scholar] [CrossRef] [Green Version]
- Lelliott, P.M.; Momota, M.; Shibahara, T.; Lee, M.S.J.; I Smith, N.; Ishii, K.J.; Coban, C. Heparin induces neutrophil elastase dependent vital and lytic NET formation. Int. Immunol. 2019. [Google Scholar] [CrossRef]
- Pretorius, E.; Bronkhorst, P.; Briedenhann, S.; Smit, E.; Franz, R.C. Comparisons of the fibrin networks during pregnancy, nonpregnancy and pregnancy during dysfibrinogenaemia using the scanning electron microscope. Blood Coagul. Fibrinolysis 2009, 20, 12–16. [Google Scholar] [CrossRef]
- Garley, M.; Jabłońska, E.; Dąbrowska, D. NETs in cancer. Tumour Biol. 2016, 37, 14355–14361. [Google Scholar] [CrossRef]
- Giaglis, S.; Stoikou, M.; Sur Chowdhury, C.; Schaefer, G.; Grimolizzi, F.; Rossi, S.W.; Hoesli, I.M.; Lapaire, O.; Hasler, P.; Hahn, S. Multimodal Regulation of NET Formation in Pregnancy: Progesterone Antagonizes the Pro-NETotic Effect of Estrogen and G-CSF. Front. Immunol. 2016, 7, 565. [Google Scholar] [CrossRef] [Green Version]
- Biegus, J.; Zymliński, R.; Gajewski, P.; Sokolski, M.; Siwołowski, P.; Sokolska, J.; Swoboda, K.; Banasiak, M.; Banasiak, W.; Ponikowski, P. Persistent hyperlactataemia is related to high rates of in-hospital adverse events and poor outcome in acute heart failure. Kardiol. Pol. 2019, 77, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variable | Acute PE Patients, n = 126 | Lactate ≥2 mM n = 70 (55.6%) | Lactate <2 mM n = 56 (44.4%) | p-Value |
|---|---|---|---|---|
| Age, years | 58.2 ± 14.4 | 60.9 ± 13.3 | 54.9 ± 15.2 | 0.02 |
| Sex (male), n (%) | 66 (52.4) | 39 (55.7) | 27 (48.2) | 0.40 |
| Body-mass index, kg/m2 | 28.1 ± 5.1 | 27.6 ± 4.6 | 28.8 ± 5.6 | 0.20 |
| Current smokers, n (%) | 25 (19.8) | 15 (21.4) | 10 (17.9) | 0.62 |
| Comorbidities and medications | ||||
| Time from PE symptom onset, days | 4 (2–7) | 3 (1–7) | 5 (2.5–7) | 0.08 |
| Low risk PE, n (%) | 20 (15.9%) | 8 (11.4) | 12 (21.4) | 0.13 |
| Intermediate–low risk PE, n (%) | 77 (61.1%) | 39 (55.7) | 38 (67.9) | 0.17 |
| Intermediate–high risk PE, n (%) | 29 (22.2%) | 23 (32.9) | 6 (10.7) | 0.0033 |
| First ever PE, n (%) | 117 (92.9) | 66 (94.3) | 51 (91.1) | 0.49 |
| Concomitant DVT, n (%) | 71 (56.3) | 43 (61.4) | 28 (50) | 0.20 |
| Coronary heart disease, n (%) | 50 (39.7) | 34 (48.6) | 16 (28.6) | 0.02 |
| Hypertension, n (%) | 68 (54) | 38 (54.3) | 30 (53.6) | 0.94 |
| Heart failure, n (%) | 25 (19.8) | 18 (25.7) | 7 (12.5) | 0.07 |
| Diabetes mellitus, n (%) | 42 (33.3) | 28 (40) | 14 (25) | 0.08 |
| Aspirin use, n (%) | 40 (31.7) | 26 (37.1) | 14 (25) | 0.15 |
| Statins use, n (%) | 77 (61.1) | 44 (62.9) | 33 (58.9) | 0.65 |
| Laboratory investigations | ||||
| White blood cell count, 103/µL | 7.03 (5.50–9.18) | 8.20 (6.40–10.35) | 5.99 (5.02–7.50) | <0.0001 |
| Neutrophil count, 103/µL | 3.82 (3.10–5.77) | 4.14 (3.55–6.69) | 3.21 (2.59–4.06) | <0.0001 |
| Hemoglobin, g/dL | 13.8 ± 1.6 | 13.9 ± 1.5 | 13.6 ± 1.6 | 0.29 |
| Platelet count, 103/µL | 220 (191–283) | 221 (198–293) | 218 (188–266) | 0.65 |
| Fibrinogen, g/L | 3.26 (2.76–3.88) | 3.35 (2.78–4.10) | 3.17 (2.73–3.62) | 0.09 |
| High-sensitivity CRP, mg/L | 3.65 (1.70–12.50) | 3.51 (1.78–14.91) | 4.20 (1.77–9.38) | 0.65 |
| D-dimer, ng/mL | 3233 (1661–5325) | 3102 (1622–5293) | 3233 (1742–5642) | 0.80 |
| NT-proBNP, pg/mL | 399 (106–1045) | 491 (135–1261) | 253 (92–742) | 0.07 |
| High-sensitivity troponin T, pg/mL | 7.3 (1–51.4) | 12 (6–78.4) | 6.5 (1–40) | 0.16 |
| Citrullinated histone H3, ng/mL | 2.77 (1.90–3.98) | 3.40 (2.03–4.31) | 2.35 (1.88–3.34) | 0.010 |
| Variable | Acute PE Patients, n = 126 | Lactate ≥2 mM n = 70 (55.6%) | Lactate <2 mM n = 56 (44.4%) | p-Value | |
|---|---|---|---|---|---|
| P-selectin, ng/mL | 77.1 ± 22.8 | 77.7 ± 23.3 | 76.5 ± 22.5 | 0.77 | |
| Platelet factor 4, ng/mL | 69.4 ± 16.9 | 69.2 ± 17.3 | 69.7 ± 16.5 | 0.85 | |
| PAI-1, ng/mL | 22.9 (16.7–33.2) | 24.6 (17.3–35.4) | 20.8 (15.8–30.3) | 0.11 | |
| TAFI activity, % | 100 (91–110) | 100 (91–110) | 100 (92–110) | 0.63 | |
| α2-antiplasmin, % | 104 ± 14 | 106 ± 16 | 102 ± 12 | 0.11 | |
| Plasminogen, % | 108 ± 15 | 112 ± 16 | 103 ± 13 | 0.0013 | |
| ETP, nM × min | 1660 (1494–1894) | 1763 (1510–2096) | 1590 (1460–1742) | 0.013 | |
| Ks, ×10–9 cm2 | 6.50 (5.46–7.40) | 6.19 (5.20–7.10) | 6.95 (5.95–7.50) | 0.026 | |
| CLT, min | 106.5 (95.0–121.6) | 111.5 (98–128) | 99.0 (88.5–113.5) | 0.003 | |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p-Value | |
| Age (per 1 year) | 1.025 (0.995–1.056) | 0.11 | - | |
| Male sex | 0.880 (0.394–1.964) | 0.65 | - | |
| BMI (per unit) | 1.101 (1.015–1.193) | 0.02 | 1.136 (1.032–1.252) | 0.01 |
| Intermediate–high PE risk | 5.042 (0.056–12.363) | 0.0004 | 3.197 (1.174–8.703) | 0.023 |
| RV dysfunction | 2.540 (1.118–5.772) | 0.026 | - | |
| hsCRP (per unit) | 1.022 (1.005–1.039) | 0.93 | 1.020 (1.002–1.038) | 0.031 |
| L-lactate (per unit) | 2.416 (1.238–4.713) | 0.0097 | 3.074 (1.351–6.992) | 0.007 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ząbczyk, M.; Natorska, J.; Janion-Sadowska, A.; Malinowski, K.P.; Janion, M.; Undas, A. Elevated Lactate Levels in Acute Pulmonary Embolism Are Associated with Prothrombotic Fibrin Clot Properties: Contribution of NETs Formation. J. Clin. Med. 2020, 9, 953. https://doi.org/10.3390/jcm9040953
Ząbczyk M, Natorska J, Janion-Sadowska A, Malinowski KP, Janion M, Undas A. Elevated Lactate Levels in Acute Pulmonary Embolism Are Associated with Prothrombotic Fibrin Clot Properties: Contribution of NETs Formation. Journal of Clinical Medicine. 2020; 9(4):953. https://doi.org/10.3390/jcm9040953
Chicago/Turabian StyleZąbczyk, Michał, Joanna Natorska, Agnieszka Janion-Sadowska, Krzysztof P. Malinowski, Marianna Janion, and Anetta Undas. 2020. "Elevated Lactate Levels in Acute Pulmonary Embolism Are Associated with Prothrombotic Fibrin Clot Properties: Contribution of NETs Formation" Journal of Clinical Medicine 9, no. 4: 953. https://doi.org/10.3390/jcm9040953
APA StyleZąbczyk, M., Natorska, J., Janion-Sadowska, A., Malinowski, K. P., Janion, M., & Undas, A. (2020). Elevated Lactate Levels in Acute Pulmonary Embolism Are Associated with Prothrombotic Fibrin Clot Properties: Contribution of NETs Formation. Journal of Clinical Medicine, 9(4), 953. https://doi.org/10.3390/jcm9040953