Isolated Exercise-Induced Pulmonary Hypertension Associates with Higher Cardiovascular Risk in Scleroderma Patients
Abstract
:1. Introduction
2. Methods
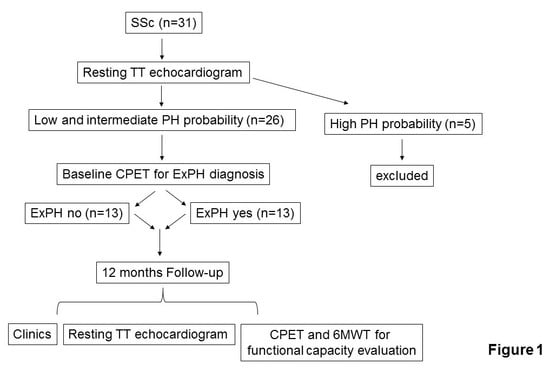
2.1. Study Design and Data Collection
2.2. Echocardiography
2.3. Cardiopulmonary Exercise Test
2.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed By: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Badesch, D.B.; Champion, H.C.; Sánchez, M.A.G.; Hoeper, M.M.; Loyd, J.; Manes, A.; McGoon, M.; Naeije, R.; Olschewski, H.; Oudiz, R.J.; et al. Diagnosis and Assessment of Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2009, 54, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; McLaughlin, V. Screening and Early Detection of Pulmonary Arterial Hypertension in Connective Tissue Diseases. It Is Time to Institute It! Am. J. Respir. Crit. Care Med. 2015, 192, 1032–1033. [Google Scholar] [CrossRef] [PubMed]
- Taboada, D.; Pepke-Zaba, J.; Jenkins, D.P.; Berman, M.; Treacy, C.M.; Cannon, J.E.; Toshner, M.; Dunning, J.J.; Ng, C.; Tsui, S.S.; et al. Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur. Respir. J. 2014, 44, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Tolle, J.J.; Waxman, A.B.; Van Horn, T.L.; Pappagianopoulos, P.P.; Systrom, D.M. Exercise-induced pulmonary arterial hypertension. Circulation 2008, 118, 2183–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madonna, R.; Bonitatibus, G.; Vitulli, P.; Pierdomenico, S.D.; Galiè, N.; De Caterina, R. Association of the European Society of Cardiology echocardiographic probability grading for pulmonary hypertension with short and mid-term clinical outcomes after heart valve surgery. Vasc. Pharmacol. 2020, 126, 106648. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic S.; American College of Chest P. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care. Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Belardinelli, R. Il Test da Sforzo Cardiopolmonare; Manuale di interpretazione: Midia, Monza, Italy, 2006. [Google Scholar]
- Wassermann, K. Cardiopulmonary Exercise Testing and Cardiovascular Health, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2002. [Google Scholar]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M.; et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J. Am. Soc. Echocardiogr. 2009, 22, 1–23; [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Moura, L.; Popescu, B.A.; Agricola, E.; Monin, J.-L.; Pierard, L.A.; Badano, L.P.; Zamorano, J.L.; et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 1: Aortic and pulmonary regurgitation (native valve disease). Eur. J. Echocardiogr. 2010, 11, 223–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, J.E.; Casaburi, R.; Cooper, D.M.; Wasserman, K. Oxygen uptake as related to work rate increment during cycle ergometer exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 1988, 57, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.; Arena, R.; Wasserman, K.; Hansen, J.E.; Lewis, G.D.; Myers, J.; Chronos, N.; Boden, W.E. Exercise-Induced Myocardial Ischemia Detected by Cardiopulmonary Exercise Testing. Am. J. Cardiol. 2009, 103, 615–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faoro, V.; Forton, K. Pulmonary Vascular Reserve and Aerobic Exercise Capacity. In Interventional Pulmonology and Pulmonary Hypertension—Updates on Specific Topics; IntechOpen: London, UK, 2019. [Google Scholar]
- Gruenig, E.; Barner, A.; Bell, M.; Claussen, M.; Dandel, M.; Dumitrescu, D.; Gorenflo, M.; Holt, S.; Kovács, G.; Ley, S.; et al. Non-invasive diagnosis of pulmonary hypertension: ESC/ERS Guidelines with Updated Commentary of the Cologne Consensus Conference 2011. Int. J. Cardiol. 2011, 154, S3–S12. [Google Scholar] [CrossRef]

| Risk Factors | Score |
|---|---|
| Clinical Signs of Heart Failure | |
| Absent | 1 |
| Present | 3 |
| Syncope | |
| No | 1 |
| Occasional | 2 |
| Repeated | 3 |
| Word Health Organization Functional Class | |
| I, II | 1 |
| III | 2 |
| IV | 3 |
| 6-Minute Walking Test | |
| >440 m | 1 |
| 165–440 m | 2 |
| <165 m | 3 |
| Peak VO2 | |
| >15 mL/min/Kg | 1 |
| 11–15 mL/min/Kg | 2 |
| <11 mL/min/Kg | 3 |
| VE/VCO2 Slope | |
| <36 | 1 |
| 18–26 cm2 | 2 |
| >26 cm2 | 3 |
| Pericardial Effusion | |
| No | 1 |
| Minimal | 2 |
| Clearly present | 3 |
| All Patients | Without ExPH | With ExPH | p | |
|---|---|---|---|---|
| (n = 26) | (n = 13) | (n = 13) | ||
| Age, y | 69 ± 13 | 68 ± 12 | 69 ± 11 | 0.975 |
| Height, cm | 160 ± 5 | 157 ± 4 | 163 ± 5 | 0.005 |
| Weight, kg | 62 ± 10 | 62 ± 11 | 63 ± 10 | 0.837 |
| BSA (m2) | 1.6 ± 0.1 | 1.67 ± 0,1 | 1.58 ± 0,1 | 0.116 |
| WHO-Functional Class, % (n) | ||||
| I/II | 85 (22) | 50 (13) | 35 (9) | 0.123 |
| III | 15 (4) | 0 (0) | 15 (4) | 0.01 |
| Physiological Parameters | ||||
| HR (beats/min) | 69 ± 6 | 68 ± 4 | 69 ± 7 | 0.828 |
| SBP (mmHg) | 120 ± 3 | 119 ± 9 | 121 ± 10 | 0.574 |
| Co-morbidities, % (n) | ||||
| Systemic arterial hypertension | 15 (4) | 4 (1) | 11 (3) | 0.22 |
| 6MWT (m) | 522 ± 139 | 637 ± 56 | 406 ± 92 | 0.0001 |
| CPET | ||||
| VO2 peak (% predicted value) | 67 ± 22 | 85 ± 4 | 48 ± 16 | 0.0001 |
| ΔVO2/ΔW (mL/min/W) | 12 ± 4 | 16 ± 1 | 9 ± 3 | 0.0001 |
| VE/VCO2 | 27 ± 13 | 15 ± 3 | 39 ± 8 | 0.0001 |
| O2 pulse (mL/min) | 9 ± 4 | 13 ± 2 | 6 ± 0.7 | 0.0001 |
| TT Echocardiogram | ||||
| Right ventricular outflow doppler acceleration time (m/s) | 128 ± 8 | 128 ± 9 | 128 ± 8 | 0.858 |
| Inferior cava diameter (cm) | 1.6 ± 0.3 | 1.6 ± 0.3 | 1.6 ± 0.2 | 0.866 |
| Right atrial area (cm2) | 15 ± 2 | 13 ± 1 | 16 ± 2 | 0.0001 |
| sPAP (mmHg) | 32 ± 14 | 20 ± 5 | 43 ± 8 | 0.0001 |
| TRV (m/s) | 2.3 ± 0.8 | 1.5 ± 0.3 | 3 ± 0.2 | 0.0001 |
| TAPSE (mm) | 23 ± 4 | 24 ± 3 | 21 ± 4 | 0.031 |
| LVEF (%) | 63 ± 4 | 64 ± 3 | 62 ± 4 | 0.121 |
| Conventional Therapies | ||||
| ACEi/ARBs | 15 (4) | 4 (1) | 11 (3) | 0.277 |
| Diuretics | 8 (2) | 0 (0) | 8 (2) | 0.141 |
| Parameters | Clinical Worsening | p | |
|---|---|---|---|
| No (n = 19) | Yes (n = 7) | ||
| Age, y | 69.7 (12.1) | 66.7 (12.3) | 0.579 |
| BMI | 24.2 (4.8) | 25.2 (2.3) | 0.607 |
| Physiological Parameters | |||
| HR (beats/min) | 70 (5) | 66 (9) | 0.222 |
| SBP (mmHg) | 121 (10) | 119 (9) | 0.583 |
| TT Echocardiogram | |||
| TAPSE (mm) | 23.2 (4) | 22.1 (4) | 0.574 |
| RV FAC (%) | 46.6 (6.0) | 50.7 (4.2) | 0.106 |
| LVEF (%) | 63 (4) | 63 (4) | 0.932 |
| Mild aortic valve regurgitation | 0.065 | ||
| no | 19 | 5 | |
| yes | 0 | 2 | |
| Score at Time 0 | 9.4 (2.4) | 9.3 (0.8) | 0.884 |
| ExPH Diagnosis | 0.005 | ||
| no | 13 | 0 | |
| yes | 6 | 7 | |
| Conventional Therapies | |||
| ACEi/ARBs | 0.287 | ||
| no | 17 | 5 | |
| yes | 2 | 2 | |
| Diuretics | 0.065 | ||
| no | 19 | 5 | |
| yes | 0 | 2 | |
| Statins | 0.999 | ||
| no | 18 | 7 | |
| yes | 1 | 0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madonna, R.; Morganti, R.; Radico, F.; Vitulli, P.; Mascellanti, M.; Amerio, P.; De Caterina, R. Isolated Exercise-Induced Pulmonary Hypertension Associates with Higher Cardiovascular Risk in Scleroderma Patients. J. Clin. Med. 2020, 9, 1910. https://doi.org/10.3390/jcm9061910
Madonna R, Morganti R, Radico F, Vitulli P, Mascellanti M, Amerio P, De Caterina R. Isolated Exercise-Induced Pulmonary Hypertension Associates with Higher Cardiovascular Risk in Scleroderma Patients. Journal of Clinical Medicine. 2020; 9(6):1910. https://doi.org/10.3390/jcm9061910
Chicago/Turabian StyleMadonna, Rosalinda, Riccardo Morganti, Francesco Radico, Piergiusto Vitulli, Marco Mascellanti, Paolo Amerio, and Raffaele De Caterina. 2020. "Isolated Exercise-Induced Pulmonary Hypertension Associates with Higher Cardiovascular Risk in Scleroderma Patients" Journal of Clinical Medicine 9, no. 6: 1910. https://doi.org/10.3390/jcm9061910
APA StyleMadonna, R., Morganti, R., Radico, F., Vitulli, P., Mascellanti, M., Amerio, P., & De Caterina, R. (2020). Isolated Exercise-Induced Pulmonary Hypertension Associates with Higher Cardiovascular Risk in Scleroderma Patients. Journal of Clinical Medicine, 9(6), 1910. https://doi.org/10.3390/jcm9061910