Meteorological Conditions in a Temperate Climate for Colletotrichum acutatum, Strawberry Pathogen Distribution and Susceptibility of Different Cultivars to Anthracnose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Colletotrichum sp. Isolate
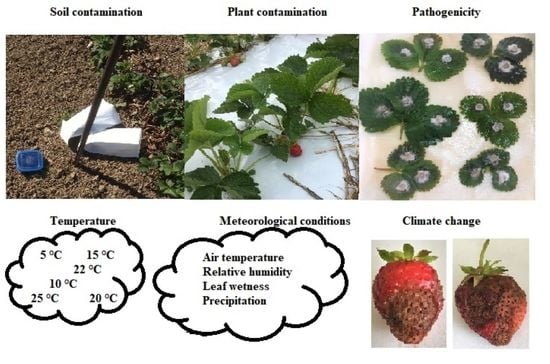
2.2. iMETOS® Meteorological Conditions
2.3. Strawberry Plant Contamination
2.4. Soil Contamination
2.5. Effect of Temperature of Growth C. acutatum
2.6. Strawberry Cultivars’ Susceptibility to C. acutatum
2.7. Statistical Analysis
3. Results
3.1. iMETOS® Meteorological Station Conditions
3.2. Strawberry Contamination
3.3. Soil Contamination
3.4. Effect of Temperature on Colletotrichum acutatum Growth
3.5. Susceptibility of Strawberries to C. acutatum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Simpson, D. The economic importance of strawberry crops Chapter 1. In The Genomes of Rosaceous Berries and Their Wild Relatives; Compendium of Plant Genomes; Hytönen, T., Graham, J., Harrison, R., Eds.; Springer International Publishing: Charm, Switzerland, 2018; pp. 1–7. ISBN 9783319760209. [Google Scholar]
- Jayawardena, R.S.; Huang, J.K.; Jin, B.C.; Yan, J.Y.; Li, X.H.; Hyde, K.D.; Bahkali, A.H.; Yin, S.L.; Zhang, G.Z. An account of Colletotrichum species associated with strawberry anthracnose in china based on morphology and molecular data. Mycosphere 2016, 7, 1147–1163. [Google Scholar] [CrossRef]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum—current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef] [Green Version]
- Udayanga, D.; Manamgoda, D.S.; Liu, X.; Chukeatirote, E.; Hyde, K.D. What are the common anthracnose pathogens of tropical fruits? Fungal Divers. 2013, 61, 165–179. [Google Scholar] [CrossRef]
- Feil, W.S.; Butler, E.E.; Duniway, J.M.; Gubler, W.D. The effects of moisture and temperature on the survival of Colletotrichum acutatum on strawberry residue in soil. Can. J. Plant Pathol. 2003, 25, 362–370. [Google Scholar] [CrossRef]
- Wagner, A.; Hetman, B. Susceptibility of strawberry cultivars to Colletotrichum acutatum J. H. Simmonds. Acta Sci. Pol. Hortorum Cultus 2013, 15, 209–219. [Google Scholar]
- Zhang, L.; Song, L.; Xu, X.; Zou, X.; Duan, K.; Gao, Q. Characterisation and fungicide sensitivity of Colletotrichum species causing strawberry anthracnose in Eastern China. Plant Dis. 2019, 104, 1960–1968. [Google Scholar] [CrossRef]
- Peres, N.A.; Timmer, L.W.; Adaskaveg, J.E.; Correll, J.C. Lifestyles of Colletotrichum acutatum. Plant Dis. 2005, 89, 784–796. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, S.; Yarden, O.; Zveibil, A.; Freeman, S. Development of a robust screening method for pathogenicity of Colletotrichum spp. on strawberry seedlings enabling forward genetic studies. Plant Dis. 2004, 88, 845–851. [Google Scholar] [CrossRef] [Green Version]
- Casado-Diaz, A.; Encinas-Villarejoa, S.; Santos, B.; Schiliro, E.; Yubero-Serrano, E.M.; Amil-Ruíz, F.; Pocovi, M.I.; Pliego-Alfaro, F.; Dorado, G.; Rey, M.; et al. Analysis of strawberry genes differentially expressed in response to Colletotrichum infection. Physiol. Plant. 2006, 128, 633–650. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Zhang, L.Q.; Song, L.L.; Duan, K.; Li, N.; Wang, Y.X.; Gao, Q.H. The different interactions of Colletotrichum gloeosporioides with two strawberry varieties and the involvement of salicylic acid. Hortic. Res. 2016, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chandra, A.; Keizerweerd, A.T.; Que, Y.; Grisham, M.P. Loop-mediated isothermal amplification (lamp) based detection of Colletotrichum falcatum causing red rot in sugarcane. Mol. Biol. Rep. 2015, 42, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Forcelini, B.B.; Seijo, T.E.; Amiri, A.; Peres, N.A. Resistance in strawberry isolates of Colletotrichum acutatum from Florida to quinone-outside inhibitor fungicides. Plant Dis. 2016, 100, 2050–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Ehsani, R.; Shi, Y.; Abdulridha, J.; Castro, A.I.; Xu, Y. Field detection of anthracnose crown rot in strawberry using spectroscopy technology. Comput. Electron. Agric. 2017, 135, 289–299. [Google Scholar] [CrossRef]
- He, L.; Li, X.; Gao, Y.; Li, B.; Mu, W.; Liu, F. Characterisation and fungicide sensitivity of Colletotrichum spp. from different hosts in Shandong, China. Plant Dis. 2019, 103, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Anciro, A.; Mangandi, J.; Verma, S.; Peres, N.; Whitaker, V.M.; Lee, S. FaRCg1: A quantitative trait locus conferring resistance to Colletotrichum crown rot caused by Colletotrichum gloeosporioides in octoploid strawberry. Theor. Appl. Genet. 2018, 131, 2167–2177. [Google Scholar] [CrossRef]
- Wang, Y.; Kerns, J.P. Temperature effects on formation of appressoria and sporulation of Colletotrichum cereale on two turfgrass species. Plant Pathol. 2017, 13, 123–132. [Google Scholar]
- Pavan, W.; Fraisse, C.W.; Cordova, L.G.; Peres, N.A. Development of the web-based disease forecasting system for strawberries. Comput. Electron. Agric. 2011, 75, 169–175. [Google Scholar] [CrossRef]
- Pavan, W.; Fraisse, C.W.; Cordova, L.G.; Peres, N.A. The Strawberry Advisory System: A Web-Based Decision Support Tool for Timing Fungicide Applications in Strawberry. Technical Report. The Agricultural and Biological Engineering Department, University of Florida, 2009; Bulletin AE450, pp. 1–4. Available online: http://cloud.agroclimate.org/tools/deprecated/sas/publications/AE45000_SAS%202012.pdf (accessed on 9 June 2020).
- Garrett, K.A.; Nita, M.; Wolf, E.D.; Esker, P.D.; Gomez-Montano, L.; Sparks, A.H. Chapter 21—Plant pathogens as indicators of climate change. In Climate Change, 2nd ed.; Observed Impacts on Planet Earth. P.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 325–338. [Google Scholar]
- Rasiukevičiūtė, N.; Valiuškaitė, A.; Survilienė-Radzevičė, E.; Supronienė, S. Investigation of Botrytis cinerea risk forecasting model of strawberry in Lithuania. In Proceedings of the Latvian Academy of Sciences, Section B. Natural, Exact and Applied Sciences; Rashal, I., Legzdiņa, A., Eds.; Sciendo: Riga, Latvia, 2013; Volume 67, pp. 195–198. [Google Scholar]
- Rasiukevičiūtė, N.; Uselis, N.; Valiuškaitė, A. The use of forecasting model IMETOS® for strawberry grey mould management. Zemdirb. Agric. 2019, 106, 143–150. [Google Scholar] [CrossRef]
- Cordova, L.G.; Ellis, M.A.; Wilson, L.L.; Madden, L.V.; Peres, N.A. Evaluation of the Florida strawberry advisory system for control Botrytis and Anthracnose fruit rots in Ohio. Plant Health Prog. 2018, 19, 182–187. [Google Scholar] [CrossRef]
- Swett, C.L.; Butler, B.B.; Peres, N.A.; Koivunen, E.E.; Hellman, E.M.; Beaulieu, J.R. Using model-based fungicide programming to effectively control Botrytis and Anthracnose fruit rots in Mid-Atlantic strawberry fields and co-manage strawberry sap beetle (Stelidota geminate). Crop Prot. 2020, 134, 1–10. [Google Scholar] [CrossRef]
- Valiuškaitė, A.; Raudonis, L.; Survilienė, E. Control of grey mould and white leaf spot in strawberry. Zemdirb. Agric. 2008, 95, 221–226. [Google Scholar]
- Valiuškaitė, A.; Uselis, N.; Kviklys, D.; Lanauskas, J.; Rasiukevičiūtė, N. The effect of sustainable plant protection and apple tree management on fruit quality and yield. Zemdirb. Agric. 2017, 104, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Subbarao, K.V.; Kabir, Z.; Martin, F.N.; Koike, S.T. Management of soilborne diseases in strawberry using vegetable rotations. Plant Dis. 2007, 91, 964–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feliziani, E.; Romanazzi, G. Postharvest decay of strawberry fruit: Etiology, epidemiology, and disease management. J. Berry Res. 2016, 6, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Dziedzinska, R.; Vasickova, P.; Hrdy, J.; Slany, M.; Babak, V.; Moravkova, M. Foodborne bacterial, viral, and protozoan pathogens in field and market strawberries and environment of strawberry farms. J. Food Sci. 2018, 83, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Carisse, O.; Morissette-Thomas, V.; Van der Heyden, H. Lagged association between powdery mildew leaf severity, airborne inoculum, weather, and crop losses in strawberry. Phytopathology 2013, 103, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Aguado, A.; Pastrana, A.M.; Santos, B.; Romero, F.; Sánchez, M.C.; Capote, N. The efficiency of natural products for the control of Colletotrichum acutatum monitored by real-time PCR. Acta Hortic. 2014, 1049, 329–334. [Google Scholar] [CrossRef]
- Mahilrajan, S.; Nandakumar, J.; Kailayalingam, R.; Manoharan, N.A.; Srivijeindran, S. Screening the antifungal activity of essential oils against decay fungi from palmyrah leaf handicrafts. Biol. Res. 2014, 47, 35. [Google Scholar] [CrossRef] [Green Version]
- Rasiukevičiūtė, N.; Rugienius, R.; Šikšnianienė, J.B. Genetic diversity of Botrytis cinerea from strawberry in Lithuania. Zemdirb. Agric. 2018, 105, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Zhang, J.; Wan, Y.; Hu, D. Identification of Colletotrichum spp. isolated from strawberry in Zhejiang Province and Shanghai City, China. J. Zhejiang Univ. Sci. B 2010, 11, 61–70. [Google Scholar] [CrossRef]
- Gillett, J.M.; Schilder, A.C. Environmental Requirements for Infection of Blueberry Fruit by Colletotrichum Acutatum. Acta Hortic. 2009, 810, 355–360. [Google Scholar] [CrossRef]
- Bahroun, A.; Jouseet, A.; Mhamdi, R.; Mrabet, M.; Mhadhbi, H. Anti-fungal activity of bacterial endophytes associated with legumes against Fusarium solani: Assessment of fungi soil suppressiveness and plant protection induction. Appl. Soil Ecol. 2018, 124, 131–140. [Google Scholar] [CrossRef]
- Bajpai, S.; Shukla, P.S.; Asiedu, S.; Pruski, K.; Prithiviraj, B. A biostimulant preparation brown seaweed Ascophyllum nodosum suppresses powdery mildew of strawberry. Plant Pathol. 2019, 35, 406–416. [Google Scholar]
- Cordova, L.G.; Madden, L.V.; Amiri, A.; Schnabel, G.; Peres, N.A. Meta-analysis of a web-based disease forecast system for control of Anthracnose and Botrytis fruit rots of strawberry in Southeastern United States. Plant Dis. 2017, 101, 1910–1917. [Google Scholar] [CrossRef]
- Jacobs, R.L.; Adhikari, T.B.; Pattison, J.; Yencho, G.C.; Fernandez, G.E.; Louws, F.J. Assessing rate-reducing foliar resistance to anthracnose crown rot and fruit rot in strawberry. Plant Dis. 2020, 104, 398–407. [Google Scholar] [CrossRef]



| District | Region | Farm Name | Grown Cultivars |
|---|---|---|---|
| Radviliškis distr., | North | Farm 1 | Florence, Asia, Rumba, Vibrant, Polka, Malvina |
| GPS 55.892954, 23.870937 | |||
| Šiauliai distr., | North | Farm 2 | Flair, Asia, Malvina |
| GPS 55.740328, 23.519661 | |||
| Šiauliai distr., | North | Farm 3 | Senga Sengana, Rumba, Polka, Malvina |
| GPS 55.9712991, 22.9108421 | |||
| Kaunas distr., | Central | Farm 4 | Asia, Rumba, Sonata, Malvina, Elkat, Deluxe |
| GPS 55.084567, 23.806315 |
| Infection Conditions | Farm 3 | Farm 4 | ||||||
|---|---|---|---|---|---|---|---|---|
| May | June | July | August | May | June | July | August | |
| Air temperature min-max, °C | 10.6–21.7 | 9.4–20.9 | 11.5–24.8 | 13.4–25.1 | 11.5–22.5 | 11.6–22.0 | 11.9–25.3 | 13.2–24.9 |
| Leaf wetness period, min | 0–925 | 0–430 | 0–990 | 0–1180 | 0–685 | 0–240 | 0–330 | 0–1440 |
| Total favourable days | 1 | 0 | 4 | 5 | 0 | 0 | 0 | 7 |
| Risk days (Days of the month) | 15 | 2, 3, 13, 30. | 11, 12, 14, 25, 26. | 11, 12, 15, 25, 26, 27, 29. | ||||
| Infection Conditions | Air Temperature Min-Max, °C | Leaf Wetness Period, Min | Total Favourable Days | Risk Days (Days of the Month) | |
|---|---|---|---|---|---|
| Farm 1 | May | 3.8–20.1 | 0–1375 | 4 | 23,27,28,29. |
| June | 15.1–24.7 | 0–1015 | 3 | 1,14,17. | |
| July | 12.1–22.9 | 0–1440 | 8 | 4,5,7,8,9,10,16,17. | |
| August | 13.6–21.4 | 0–990 | 6 | 8,9,10,21,22,30. | |
| Farm 2 | May | 4.3–30.8 | 0–1425 | 3 | 23,27,28. |
| June | 15.1–25.8 | 0–1000 | 2 | 1,17. | |
| July | 12.5–23.1 | 0–1430 | 8 | 5,7,8,9,10,16,17,22. | |
| August | 14.2–22.1 | 0–995 | 5 | 8,9,21,22,30. | |
| Farm 3 | May | 4.3–21.6 | 0–1345 | 0 | |
| June | 15.1–25.8 | 0–1000 | 2 | 1,17. | |
| July | 12.5–23.1 | 0–1430 | 8 | 5,7,8,9,10,11,16,17 | |
| August | 14.2–22.1 | 0–920 | 5 | 9,10,14,15,21. | |
| Farm 4 | May | 5.2–22.1 | 0–1055 | 3 | 11,29,31. |
| June | 16.4–26.3 | 0–680 | 0 | ||
| July | 12.7–22.8 | 0–1375 | 11 | 3,7,8,9,10,11,16,17,18, 22,27. | |
| August | 14–22.1 | 0–1025 | 7 | 2,9,10,11, 21,22,30. | |
| Isolated Fungi/Cultivar | Frequency of Isolated Fungi (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Rumba | Asia | Florence | Malvina | Senga Sengana | ||||||
| Mycosphaerella spp. | 0 | 0 | 0 | 0 | 0 | 0 | 21 | 13.9 | 0 | 0 |
| Alternaria spp. | 4 | 4.3 | 41 | 29.7 | 7 | 7.4 | 10 | 6.6 | 22 | 41.5 |
| Unknown | 10 | 10.9 | 27 | 19.6 | 11 | 11.7 | 27 | 17.9 | 6 | 11.3 |
| Fusarium spp. | 46 | 50 | 36 | 26.1 | 9 | 9.6 | 40 | 26.5 | 14 | 26.4 |
| Colletotrichum spp. | 2 | 2.2 | 2 | 1.5 | 3 | 3.2 | 5 | 3.3 | 1 | 1.9 |
| Mucor spp. | 1 | 1.1 | 5 | 3.6 | 0 | 0 | 14 | 9.3 | 3 | 5.7 |
| Penicillium spp. | 15 | 16.3 | 12 | 8.7 | 51 | 54.3 | 10 | 6.6 | 4 | 7.5 |
| Phytophthora spp. | 12 | 13 | 8 | 5.8 | 4 | 4.2 | 8 | 5.3 | 1 | 1.9 |
| Botrytis spp. | 0 | 0 | 2 | 1.4 | 9 | 9.6 | 3 | 2 | 0 | 0 |
| Trichoderma spp. | 2 | 2.2 | 5 | 3.6 | 0 | 0 | 13 | 8.6 | 2 | 3.8 |
| Total | 92 | 100 | 138 | 100 | 94 | 100 | 151 | 100 | 53 | 100 |
| Farm | Total Amount, CFU/g−1 | ||
|---|---|---|---|
| Cultivar | Bacteria | Fungi | |
| Farm 1 | ´Florence´ | 3.98 ± 0.69 cd | 3.60 ± 0.40 bcd |
| ´Asia´ | 4.14 ± 0.48 d | 3.44 ± 0.82 bcd | |
| ´Rumba´ | 3.92 ± 1.17 bcd | 3.24 ± 0.29 ab | |
| ´Vibrant´ | 4.13 ± 1.02 d | 3.30 ± 0.63 ab | |
| Farm 2 | ´Flair´ | 3.48 ± 0.27 abc | 3.00 ± 0.63 a |
| ´Malvina´ | 3.48 ± 0.62 abc | 3.10 ± 0.64 ab | |
| Farm 3 | ´Rumba´ | 4.05 ± 0.50 d | 3.18 ± 0.29 ab |
| ´Polka´ | 3.18 ± 0.58 ab | 2.70 ± 0.41 a | |
| ´Asia´ | 3.18 ± 0.64 ab | 3.00 ± 0.71 a | |
| Farm 4 | ´Elkat´ | 4.17 ± 0.87 d | 3.72 ± 0.91 d |
| ´Malvina´ | 2.87 ± 0.85 a | 3.60 ± 0.63 bcd | |
| ´Sonata´ | 4.49 ± 0.41 f | 2.70 ± 0.27 a | |
| ´Deluxe´ | 4.49 ± 0.65 f | 3.10 ± 0.25 ab | |
| C. acutatum Mycelium Growth, cm (Factor A) | ||||
|---|---|---|---|---|
| Temperatures, °C (Factor B) | 2DPI | 4DPI | 7DPI | Average B (p = 0.064) |
| 5 | 0.00 ± 0.00 | 0.80 ± 0.00 | 0.83 ± 0.02 | 0.54 ** |
| 10 | 0.00 ± 0.00 | 1.20 ± 0.02 | 2.44 ± 0.04 | 1.21 |
| 15 | 0.81 ± 0.02 | 1.61 ± 0.03 | 2.73 ± 0.04 | 1.71 |
| 20 | 1.25 ± 0.05 | 2.68 ± 0.05 | 4.41 ± 0.11 | 2.78 ** |
| 22 | 1.24 ± 0.11 | 2.90 ± 0.06 | 4.87 ± 0.10 | 3.00 |
| 25 | 1.76 ± 0.08 | 3.35 ± 0.05 | 6.01 ± 0.13 | 3.71 ** |
| Average A (p = 0.054) | 0.84 | 2.09 | 3.55 ** | 2.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morkeliūnė, A.; Rasiukevičiūtė, N.; Valiuškaitė, A. Meteorological Conditions in a Temperate Climate for Colletotrichum acutatum, Strawberry Pathogen Distribution and Susceptibility of Different Cultivars to Anthracnose. Agriculture 2021, 11, 80. https://doi.org/10.3390/agriculture11010080
Morkeliūnė A, Rasiukevičiūtė N, Valiuškaitė A. Meteorological Conditions in a Temperate Climate for Colletotrichum acutatum, Strawberry Pathogen Distribution and Susceptibility of Different Cultivars to Anthracnose. Agriculture. 2021; 11(1):80. https://doi.org/10.3390/agriculture11010080
Chicago/Turabian StyleMorkeliūnė, Armina, Neringa Rasiukevičiūtė, and Alma Valiuškaitė. 2021. "Meteorological Conditions in a Temperate Climate for Colletotrichum acutatum, Strawberry Pathogen Distribution and Susceptibility of Different Cultivars to Anthracnose" Agriculture 11, no. 1: 80. https://doi.org/10.3390/agriculture11010080
APA StyleMorkeliūnė, A., Rasiukevičiūtė, N., & Valiuškaitė, A. (2021). Meteorological Conditions in a Temperate Climate for Colletotrichum acutatum, Strawberry Pathogen Distribution and Susceptibility of Different Cultivars to Anthracnose. Agriculture, 11(1), 80. https://doi.org/10.3390/agriculture11010080