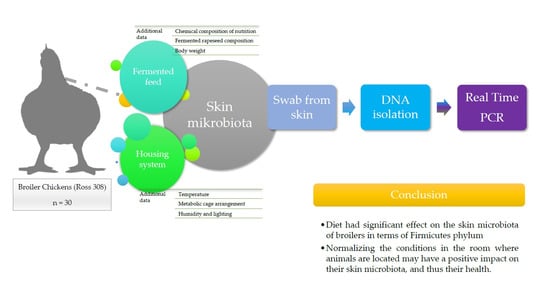
Levels of Firmicutes, Actinobacteria Phyla and Lactobacillaceae Family on the Skin Surface of Broiler Chickens (Ross 308) Depending on the Nutritional Supplement and the Housing Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
- A—control (no rapeseed meal);
- B—addition of 5% of standard rapeseed meal;
- C—addition of 10% of standard rapeseed meal;
- D—addition of 5% standard rapeseed meal and 5% fermented rapeseed meal;
- E—addition of 10% standard rapeseed meal and 10% fermented rapeseed meal.
2.2. Sampling
2.3. Assessment of the Housing Environment
2.4. DNA (Deoxyrybonucleic acid) Isolation
2.5. Real-Time PCR Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. 2017. Available online: http://www.fao.org/poultry-production-products/production/en/ (accessed on 9 January 2021).
- Cholewińska, P.; Górniak, W.; Wojnarowski, K. Impact of selected environmental factors on microbiome of the digestive tract of ruminants. BMC Vet. Res. 2021, 17, 1–10. [Google Scholar] [CrossRef]
- Weeks, C.A.; Lambton, S.L.; Williams, A.G. Implications for welfare, productivity and sustainability of the variation in reported levels of mortality for laying hen flocks kept in different housing systems: A meta-analysis of ten studies. PLoS ONE 2016, 11, e0146394. [Google Scholar] [CrossRef] [Green Version]
- Atterbury, R.J.; Gigante, A.M.; Lozano, M.D.L.S.R.; Medina, R.D.M.; Robinson, G.; Alloush, H.; Allen, V.M. Reduction of salmonella contamination on the surface of chicken skin using bacteriophage. Virol. J. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Jablonski, N.G. Skin: A Natural History; University of California Press: Berkeley, CA, USA, 2008. [Google Scholar]
- Ross, A.A.; Rodrigues Hoffmann, A.; Neufeld, J.D. The skin microbiome of vertebrates. Microbiome 2019, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Nagase, S.; Ogai, K.; Urai, T.; Shibata, K.; Matsubara, E.; Mukai, K.; Okamoto, S. Distinct skin microbiome and skin physiological functions between bedridden older patients and healthy people: A single-center study in Japan. Front. Med. 2020, 7, 101. [Google Scholar] [CrossRef]
- Hoffmann, A.R. The cutaneous ecosystem: The roles of the skin microbiome in health and its association with inflammatory skin conditions in humans and animals. Adv. Vet. Dermatol. 2017, 8, 71–83. [Google Scholar]
- Naji, S.A.H.; AI-Zanili, I.F.B.; AI-Gharawi, J. The effect of feed wetting and fermentation on the intestinal flora, humoral and cellular immunity of broiler chicks. Int. J. Adv. Res. 2015, 3, 87–94. [Google Scholar]
- Brandwein, M.; Katz, I.; Katz, A.; Kohen, R. Beyond the gut: Skin microbiome compositional changes are associated with BMI. Hum. Microbiome J. 2019, 13, 100063. [Google Scholar] [CrossRef]
- Górniak, W.; Cholewińska, P.; Szeligowska, N.; Wołoszyńska, M.; Soroko, M.; Czyż, K. Effect of intense exercise on the level of bacteroidetes and Firmicutes phyla in the digestive system of thoroughbred racehorses. Animals 2021, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Dowd, S.E.; Callaway, T.R.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Hagevoort, R.G.; Edrington, T.S. Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol. 2008, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- De Gregoris, T.B.; Aldred, N.; Clare, A.S.; Burgess, J.G. Improvement of phylum-and class-specific primers for real-time PCR quantification of bacterial taxa. J. Microbiol. Methods 2011, 86, 351–356. [Google Scholar] [CrossRef]
- Blackwood, C.B.; Oaks, A.; Buyer, J.S. Phylum-And class-specific PCR primers for general microbial community analysis. Appl. Environ. Microbiol. 2005, 71, 6193–6198. [Google Scholar] [CrossRef] [Green Version]
- Walter, J.; Hertel, C.; Tannock, G.W.; Lis, C.M.; Munro, K.; Hammes, W.P. Detection of lactobacillus, pediococcus, leuconostoc, and weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 2001, 67, 2578–2585. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Issa, N.; Afifi, L. The Role of the skin and gut microbiome in psoriatic disease. Curr. Dermatol. Rep. 2017, 6, 94–103. [Google Scholar] [CrossRef]
- Xiao, Y.; Yun, X.; Weidong, Z.; Jinggang, C.; Kaifeng, L.; Hua, Y. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017, 96, 1387–1393. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Global Rumen Census Collaborators; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.B.; Morales, C.A.; Line, J.; Berrang, M.E.; Meinersmann, R.J.; Tillman, G.E.; Wise, M.G.; Siragusa, G.R.; Hiett, K.L.; Seal, B.S. The poultry-associated microbiome: Network analysis and farm-to-fork characterizations. PLoS ONE 2013, 8, e57190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Older, C.E.; Diesel, A.B.; Lawhon, S.D.; Queiroz, C.R.; Henker, L.C.; Rodrigues Hoffmann, A. The feline cutaneous and oral microbiota are influenced by breed and environment. PLoS ONE 2019, 14, e0220463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morvan, P.Y.; Vallee, R. Evaluation of the effects of stressful life on human skin microbiota. Appl. Microbiol. Open Access 2018, 4, 1–8. [Google Scholar]
- Lehtimäki, J.; Karkman, A.; Laatikainen, T.; Paalanen, L.; Von Hertzen, L.; Haahtela, T.; Ruokolainen, L. Patterns in the skin microbiota differ in children and teenagers between rural and urban environments. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shawkey, M.D.; Mills, K.L.; Dale, C. Microbial diversity of wild bird feathers revealed through culture-based and culture-independent techniques. Microb. Ecol. 2005, 50, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Romanovsky, A.A. Skin temperature: Its role in thermoregulation. Acta Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Theurer, M.E.; White, B.J.; Anderson, D.E.; Miesner, M.D.; Mosier, D.A.; Coetzee, J.F.; Amrine, D.E. Effect of transportation during periods of high ambitne temperature on physiologic ang behavioral indices of beef heifers. Am. J. Vet. Res. 2013, 74, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Jutte, L.S.; Merrick, M.A.; Ingersoll, C.D.; Edwards, J.E. The relationship between intramuscular temperature, skin temperature, and adipose thickness during cryotherapy and rewarming. Arch. Phys. Med. Rehabil. 2001, 82, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, Z. FBA Ecological guild: Trio of firmicutes-bacteroidetes alliance against actinobacteria in human oral microbiome. Sci. Rep. 2020, 10, 287. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Qian, L.; Yuquan, W.; Hongyang, C.; Xu, Z.; Xueqin, W.; Si, S.; Zimin, W. Effect of actinobacteria agent inoculation methods on cellulose degradation during composting based on redundancy analysis. Bioresour. Technol. 2016, 219, 196–203. [Google Scholar] [CrossRef] [PubMed]






| Mixture | Group | Dry Matter (%) | Ash (%) | Protein (%) | Fat (%) | Fiber (%) | Gross Energy (kcal) |
|---|---|---|---|---|---|---|---|
| Starter (Day: 1–7) | A | 87.94 | 4.46 | 21.90 | 7.63 | 7.24 | 4275.29 |
| B | 87.90 | 3.99 | 20.77 | 7.45 | 7.39 | 4252.56 | |
| C | 87.70 | 4.17 | 22.78 | 7.81 | 7.67 | 4306.74 | |
| D | 87.63 | 4.41 | 22.37 | 7.72 | 7.53 | 4293.92 | |
| E | 87.99 | 4.83 | 24.07 | 7.41 | 7.10 | 4243.74 | |
| Grower 1 (Day: 8–28) | A | 89.17 | 6.33 | 19.98 | 8.11 | 7.62 | 4268.95 |
| B | 89.03 | 5.95 | 19.25 | 7.80 | 6.92 | 4247.90 | |
| C | 89.37 | 6.41 | 19.83 | 8.27 | 6.61 | 4246.39 | |
| D | 89.16 | 5.90 | 20.67 | 8.60 | 6.55 | 4294.93 | |
| E | 89.59 | 5.92 | 20.23 | 7.87 | 7.41 | 4308.50 | |
| Grower 2 (Day: 28–35) | A | 89.76 | 6.58 | 20.85 | 8.90 | 6.01 | 4356.83 |
| B | 89.90 | 5.98 | 20.77 | 9.89 | 6.17 | 4398.55 | |
| C | 89.58 | 6.02 | 20.22 | 9.62 | 8.11 | 4364.70 | |
| D | 89.74 | 6.40 | 20.61 | 9.02 | 6.31 | 4358.05 | |
| E | 89.57 | 6.62 | 20.90 | 8.86 | 8.61 | 4327.62 |
| Cage Position | Top/Window | Top/Door | Middle/Door | Middle/Window | Bottom/Window | Bottom/Door |
|---|---|---|---|---|---|---|
| Mean | +1.05 | +/−0.42 | +/−0.31 | +0.61 | −0.65 | −0.44 |
| SD | 0.30 | 0.28 | 0.34 | 0.15 | 0.15 | 0.35 |
| Component | Volume in 10 μL of Reaction |
|---|---|
| SsoAdvanced™ Universal SYBR® Green Supermix | 5 μL |
| Starter (Foward + Reverse) | 1 μL (0.8 μM) |
| Matrix DNA | 2 μL (0.04 − 0.015 × 10−4) |
| Sterile water | 2 μL |
| Name | Forward (5′–3′) | Reverse (5′–3′) | Source |
|---|---|---|---|
| Universal Eubacterial genes | 530F (5′-GTC CCA GCM GCN GCG G) | 1100R (5′-GGG TTN CGN TCG TTG) | [12] |
| Firmicutes | 928F-Firm (5′-TGA AAC TYA AAG GAA TTG ACG) | 1040FirmR (5′-ACC ATG CAC CTG TC) | [13] |
| Actinobacteria | Act1159R TCCGAGTTRACCCCGGC | Eub338F ACGGGCGGTGTGTACA | [14] |
| Lactobacillaceae | lac1 forward (5′-AGC AGT AGG GAA TCT TCC A) | Lac2Seq (5′-ATTTCACCGCTACACATG) | [15] |
| Group | A | B | C | D | E |
|---|---|---|---|---|---|
| Mean | 1729.92 a | 1615.83 A | 1707.29 A | 1920.76 B,b | 1642.57 A |
| SD | 79.17 | 66.05 | 129.68 | 83.98 | 33.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cholewińska, P.; Michalak, M.; Wojnarowski, K.; Skowera, S.; Smoliński, J.; Czyż, K. Levels of Firmicutes, Actinobacteria Phyla and Lactobacillaceae Family on the Skin Surface of Broiler Chickens (Ross 308) Depending on the Nutritional Supplement and the Housing Conditions. Agriculture 2021, 11, 287. https://doi.org/10.3390/agriculture11040287
Cholewińska P, Michalak M, Wojnarowski K, Skowera S, Smoliński J, Czyż K. Levels of Firmicutes, Actinobacteria Phyla and Lactobacillaceae Family on the Skin Surface of Broiler Chickens (Ross 308) Depending on the Nutritional Supplement and the Housing Conditions. Agriculture. 2021; 11(4):287. https://doi.org/10.3390/agriculture11040287
Chicago/Turabian StyleCholewińska, Paulina, Marta Michalak, Konrad Wojnarowski, Szymon Skowera, Jakub Smoliński, and Katarzyna Czyż. 2021. "Levels of Firmicutes, Actinobacteria Phyla and Lactobacillaceae Family on the Skin Surface of Broiler Chickens (Ross 308) Depending on the Nutritional Supplement and the Housing Conditions" Agriculture 11, no. 4: 287. https://doi.org/10.3390/agriculture11040287
APA StyleCholewińska, P., Michalak, M., Wojnarowski, K., Skowera, S., Smoliński, J., & Czyż, K. (2021). Levels of Firmicutes, Actinobacteria Phyla and Lactobacillaceae Family on the Skin Surface of Broiler Chickens (Ross 308) Depending on the Nutritional Supplement and the Housing Conditions. Agriculture, 11(4), 287. https://doi.org/10.3390/agriculture11040287