Comparative Effect of Melatonin and 1-Methylcyclopropene Postharvest Applications for Extending ‘Hayward’ Kiwifruit Storage Life
Abstract
:1. Introduction
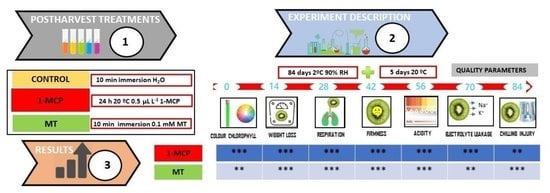
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Postharvest Quality Parameters
2.3. Statistical Analysis
3. Results and Discussions
3.1. Effect of Exogenous 1-MCP and MT on Weight Loss and Fruit Firmness
3.2. Effect of Exogenous 1-MCP and MT on Respiration Rate and Ethylene Production
3.3. Effect of Exogenous 1-MCP and MT on TSS and TA
3.4. Effect of Exogenous 1-MCP and MT on Chlorophyll Content and Internal Colour
3.5. Effect of Exogenous 1-MCP and MT on EL and Chilling Injury
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Satpal, D.; Kaur, J.; Bhadariya, V.; Sharma, K. Actinidia deliciosa (Kiwi fruit): A comprehensive review on the nutritional composition, health benefits, traditional utilization, and commercialization. J. Food Process. Preserv. 2021, 45, e15588. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Zhu, F. Kiwifruit (Actinidia spp.): A review of chemical diversity and biological activities. Food Chem. 2021, 350, 128469. [Google Scholar] [CrossRef]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, X.; Wang, J.; Vidyarthi, S.K.; Wang, H.; Zhang, X.G.; Gao, L.; Yang, K.W.; Zhang, J.S.; Xiao, H.W. Effects of postharvest ripening on water status and distribution, drying characteristics, volatile profiles, phytochemical contents, antioxidant capacity and microstructure of kiwifruit (Actinidia deliciosa). Food Control 2022, 139, 109062. [Google Scholar] [CrossRef]
- Yi, J.; Kebede, B.T.; Grauwet, T.; Loey, A.V.; Hu, X.; Hendrickx, M. A multivariate approach into physicochemical, biochemical and aromatic quality changes of purée based on ’Hayward’ kiwifruit during the final phase of ripening. Postharvest Biol. Technol. 2016, 117, 206–216. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Z.; Leng, J.; Sui, Y.; Jiang, M.; Wisniewski, M.; Liu, J.; Wang, Q. Eco-friendly management of postharvest fungal decays in kiwifruit. Crit. Rev. Food Sci. Nutr. 2022, 62, 8307–8318. [Google Scholar] [CrossRef]
- Mitalo, O.W.; Tokiwa, S.; Kondo, Y.; Otsuki, T.; Galis, I.; Suezawa, K.; Kataoka, I.; Doan, A.T.; Nakano, R.; Ushijima, K.; et al. Low temperature storage stimulates fruit softening and sugar accumulation without ethylene and aroma volatile production in kiwifruit. Front. Plant Sci. 2019, 10, 888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, G.H.; Kumarihami, H.P.C.; Kim, H.L.; Kwack, Y.B.; Kim, J.G. Storage temperature influences fruit ripening and changes in organic acids of kiwifruit treated with exogenous ethylene. Hortic. Sci. Technol. 2019, 37, 618–629. [Google Scholar]
- Chai, J.; Wang, Y.; Liu, Y.; Gu, Z.; Liu, Z. High O2/N2 controlled atmosphere accelerates postharvest ripening of ‘Hayward’ kiwifruit. Sci. Hortic. 2022, 300, 111073. [Google Scholar] [CrossRef]
- Han, Y.; East, A.; Nicholson, S.; Jeffery, P.; Glowacz, M.; Heyes, J. Benefits of modified atmosphere packaging in maintaining ‘Hayward’ kiwifruit quality at room temperature retail conditions. N. Z. J. Crop Hortic. Sci. 2022, 50, 242–258. [Google Scholar] [CrossRef]
- Kim, K.H.; Yook, H.S. Effect of gamma irradiation on quality of kiwifruit (Actinidia deliciosa var. deliciosa cv. Hayward). Radiat. Phys. Chem. 2009, 78, 414–421. [Google Scholar] [CrossRef]
- Kumarihami, H.P.C.; Kim, Y.H.; Kwack, Y.B.; Kim, J.; Kim, J.G. Application of chitosan as edible coating to enhance storability and fruit quality of Kiwifruit: A Review. Sci. Hortic. 2022, 292, 110647. [Google Scholar] [CrossRef]
- Öztürk, B.; Yücedağ, F. Effects of methyl jasmonate on quality properties and phytochemical compounds of kiwifruit (Actinidia deliciosa cv. Hayward) during cold storage and shelf life. Turk. J. Agric. For. 2021, 45, 154–164. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Jin, S.; Dong, W.; Zhang, Y.; Chen, W.; Shi, L.; Cao, S.; Yang, Z. γ-Aminobutyric acid treatment induced chilling tolerance in postharvest kiwifruit (Actinidia chinensis cv. Hongyang) via regulating ascorbic acid metabolism. Food Chem. 2023, 404, 134661. [Google Scholar] [CrossRef]
- Minas, I.S.; Karaoglanidis, G.S.; Manganaris, G.A.; Vasilakakis, M. Effect of ozone application during cold storage of kiwifruit on the Development of stem-end rot caused by Botrytis cinerea. Postharvest Biol. Technol. 2010, 58, 203–210. [Google Scholar] [CrossRef]
- Luo, A.; Bai, J.; Li, R.; Fang, Y.; Li, L.; Wang, D.; Zhang, L.; Liang, J.; Huang, T.; Kou, L. Effects of ozone treatment on the quality of kiwifruit during postharvest storage affected by Botrytis cinerea and Penicillium expansum. J. Phytopathol. 2019, 167, 470–478. [Google Scholar] [CrossRef]
- Cantin, C.M.; Holcroft, D.; Crisosto, C.H. Postharvest application of 1-methylcyclopropene (1-MCP) extends shelf life of kiwifruit. Acta Hortic. 2011, 913, 621–626. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S.; Liu, Y.; Xu, J.; Liu, T.; Dong, S. Effectiveness of lysozyme coatings and 1-MCP treatments on storage and preservation of kiwifruit. Food Chem. 2019, 288, 201–207. [Google Scholar] [CrossRef]
- Krupa, T.; Tomala, K.; Zaraś-Januszkiewicz, E. Evaluation of Storage Quality of ‘Hardy’ Kiwifruit (Actinidia arguta): Effect of 1-MCP and Maturity Stage. Agriculture 2022, 12, 2062. [Google Scholar] [CrossRef]
- Cornacchia, R.; Amodio, M.L.; Rinaldi, R.; Colelli, G. Effect of 1-methylcyclopropene and controlled atmosphere on storage of kiwifruits. Fresh Prod. 2008, 2, 22–25. [Google Scholar]
- Koukounaras, A.; Sfakiotakis, E. Effect of 1-MCP prestorage treatment on ethylene and CO2 production and quality of ‘Hayward’ kiwifruit during shelf-life after short, medium and long term cold storage. Postharvest Biol. Technol. 2007, 46, 174–180. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Boquete, E.J.; Trinchero, G.D.; Fraschina, A.A.; Vilella, F.; Sozzi, G.O. Ripening of ‘Hayward’ kiwifruit treated with 1-methylcyclopropene after cold storage. Postharvest Biol. Technol. 2004, 32, 57–65. [Google Scholar] [CrossRef]
- Salazar, J.; Jorquera, C.; Campos-Vargas, R.; Jorgensen, C.; Zapata, P.; Infante, R. Effect of the application timing of 1-MCP on postharvest traits and sensory quality of a yellow-fleshed kiwifruit. Sci. Hortic. 2019, 244, 82–87. [Google Scholar] [CrossRef]
- Algul, B.E.; Al Shoffe, Y.; Park, D.; Miller, W.B.; Watkins, C.B. Preharvest 1-methylcyclopropene treatment enhances ‘stress-associated watercore’ dissipation in ‘Jonagold’ apples. Postharvest Biol. Technol. 2021, 181, 111689. [Google Scholar] [CrossRef]
- Huang, H.; Guo, L.; Wang, L.; Wang, H.; Ma, S.; Jiang, Y.; Qu, H. 1-Methylcyclopropene (1-MCP) slows ripening of kiwifruit and affects energy status, membrane fatty acid contents and cell membrane integrity. Postharvest Biol. Technol. 2019, 156, 110941. [Google Scholar] [CrossRef]
- Park, Y.S.; Im, M.H.; Gorinstein, S. Shelf-life extension and antioxidant activity of ‘Hayward’ kiwifruit as a result of prestorage conditioning and 1-methylcyclopropene treatment. J. Food Sci. Technol. 2015, 52, 2711–2720. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Han, S.H.; Kim, J.; Lee, H.J.; Lee, J.G.; Lee, E.J. Inhibition of hardy kiwifruit (Actinidia aruguta) ripening by 1-methylcyclopropene during cold storage and anticancer properties of the fruit extract. Food Chem. 2016, 190, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Huan, C.; Zhang, J.; Jia, Y.; Jiang, T.; Shen, S.; Zheng, X. Effect of 1-methylcyclopropene treatment on quality, volatile production and ethanol metabolism in kiwifruit during storage at room temperature. Sci. Hortic. 2020, 265, 109266. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Dole, J.M. 1-Methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Q.; Li, J.; Gong, H.; Fan, X.; Yang, Y.; Liu, X.; Yin, X. Transcriptome co-expression network analysis identifies key genes and regulators of ripening kiwifruit ester biosynthesis. BMC Plant Biol. 2020, 20, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef] [Green Version]
- Madebo, M.P.; Hu, S.; Zheng, Y.; Jin, P. Mechanisms of chilling tolerance in melatonin treated postharvest fruits and vegetables: A review. J. Future Foods 2021, 1, 156–167. [Google Scholar] [CrossRef]
- Cao, S.; Qu, G.; Ma, C.; Ba, L.; Ji, N.; Meng, L.; Lei, J.; Wang, R. Effects of melatonin treatment on the physiological quality and cell wall metabolites in kiwifruit. Food Sci. Technol. 2021, 42, e85421. [Google Scholar] [CrossRef]
- Hu, M.; Li, J.; Rao, J. Effect of melatonin on ripening and senescence of postharvest kiwifruits. Sh. Kexue/Food Sci. 2018, 39, 226–232. [Google Scholar]
- Wang, X.; Liang, D.; Xie, Y.; Lv, X.; Wang, J.; Xia, H. Melatonin application increases accumulation of phenol substances in kiwifruit during storage. Emir. J. Food Agric. 2019, 31, 361–367. [Google Scholar]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X.; et al. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Mujumdar, A.S.; Jin, X.; Liu, Z.L.; Zhang, Y.; Xiao, H.W. Effects of postharvest ripening on physicochemical properties, microstructure, cell wall polysaccharides contents (pectin, hemicellulose, cellulose) and nanostructure of kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021, 118, 106808. [Google Scholar] [CrossRef]
- Tavarini, S.; Degl’Innocenti, E.; Remorini, D.; Massai, R.; Guidi, L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of ‘Hayward’ kiwifruit. Food Chem. 2008, 107, 282–288. [Google Scholar] [CrossRef]
- Jiao, J.; Jin, M.; Liu, H.; Suo, J.; Yin, X.; Zhu, Q.; Rao, J. Application of melatonin in kiwifruit (Actinidia chinensis) alleviated chilling injury during cold storage. Sci. Hortic. 2022, 296, 110876. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, A.; Li, H.; Huan, C.; Jiang, T.; Shen, S.; Zheng, X. Effects of melatonin treatment on ethanol fermentation and ERF expression in kiwifruit cv. Bruno during postharvest. Sci. Hortic. 2022, 293, 110696. [Google Scholar] [CrossRef]
- Almeida, D.P.; Gomes, M.H. Uncoupling the sensory effects of 1-Methylcyclopropene and ripening stage on ‘Hayward’ kiwifruit. HortScience 2009, 44, 1936–1940. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Romero, D.; Serrano, M.; Carbonell, A.; Burgos, L.; Riquelme, F.; Valero, D. Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. J. Food Sci. 2002, 67, 1706–1712. [Google Scholar] [CrossRef]
- Mao, L.C.; Wang, G.Z.; Zhu, C.G.; Pang, H.Q. Involvement of phospholipase D and lipoxygenase in response to chilling stress in postharvest cucumber fruits. Plant Sci. 2007, 172, 400–405. [Google Scholar] [CrossRef]
- McCollum, T.G.; McDonald, R.E. Electrolyte leakage, respiration, and ethylene production as indices of chilling injury in grapefruit. HortScience 1991, 26, 1191–1192. [Google Scholar] [CrossRef] [Green Version]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Changes of phytochemicals and antioxidant capacity of banana peel during the ripening process; with and without ethylene treatment. Sci. Hortic. 2019, 253, 255–262. [Google Scholar] [CrossRef]
- Celano, G.; Minnocci, A.; Sebastiani, L.; D’auria, M.; Xiloyannis, C. Changes in the structure of the skin of kiwifruit in relation to water loss. J. Hortic. Sci. Biotechnol. 2009, 84, 41–46. [Google Scholar] [CrossRef]
- Burdon, J.; Clark, C. Effect of postharvest water loss on ’Hayward’ kiwifruit water status. Postharvest Biol. Technol. 2001, 22, 215–225. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Jeong, J.; Huber, D.J.; Sargent, S.A. Influence of 1-methylcyclopropene (1-MCP) on ripening and cell-wall matrix polysaccharides of avocado (Persea americana) fruit. Postharvest Biol. Technol. 2002, 25, 241–256. [Google Scholar] [CrossRef]
- Guillén, F.; Castillo, S.; Zapata, P.J.; Martínez-Romero, D.; Serrano, M.; Valero, D. Efficacy of 1-MCP treatment in tomato fruit: 1. Duration and concentration of 1-MCP treatment to gain an effective delay of postharvest ripening. Postharvest Biol. Technol. 2007, 43, 23–27. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, Y.; Chen, X.; Xu, W.; Wang, N.; Xu, F.; Wang, H.; Shao, X. Postharvest strategy combining maturity and storage temperature for 1-MCP-treated peach fruit. J. Food Process. Preserv. 2020, 44, e14388. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Onik, J.C.; Wai, S.C.; Li, A.; Lin, Q.; Sun, Q.; Wang, Z.; Duan, Y. Melatonin treatment reduces ethylene production and maintains fruit quality in apple during postharvest storage. Food Chem. 2021, 337, 127753. [Google Scholar] [CrossRef]
- Latocha, P.; Krupa, T.; Jankowski, P.; Radzanowska, J. Changes in postharvest physicochemical and sensory characteristics of hardy kiwifruit (Actinidia arguta and its hybrid) after cold storage under normal versus controlled atmosphere. Postharvest Biol. Technol. 2014, 88, 21–33. [Google Scholar] [CrossRef]
- Paniagua, A.C.; East, A.R.; Hindmarsh, J.P.; Heyes, J. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol. Technol. 2013, 79, 13–19. [Google Scholar] [CrossRef]
- Gwanpua, S.G.; Zhao, M.; Jabbar, A.; Bronlund, J.E.; East, A.R. A model for firmness and low temperature-induced storage breakdown disorder of ‘Hayward’ kiwifruit in supply chain. Postharvest Biol. Technol. 2022, 185, 111789. [Google Scholar] [CrossRef]
- Mahajan, B.V.C.; Singh, K.; Dhillon, W.S. Effect of 1-methylcyclopropene (1-MCP) on storage life and quality of pear fruits. J. Food Sci. Technol. 2010, 47, 351–354. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Liu, Y.; Dong, S.; Wang, S. Effect of 1-methylcyclopropene (1-MCP) on ripening and volatile compounds of blueberry fruit. J. Food Process. Preserv. 2020, 44, e14840. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, T.; Zhang, P.; Wang, Z.Y. Melatonin attenuates postharvest physiological deterioration of cassava storage roots. J. Pineal Res. 2016, 60, 424–434. [Google Scholar] [CrossRef]
- Sabban-Amin, R.; Feygenberg, O.; Belausov, E.; Pesis, E. Low oxygen and 1-MCP pretreatments delay superficial scald development by reducing reactive oxygen species (ROS) accumulation in stored ‘Granny Smith’apples. Postharvest Biol. Technol. 2011, 62, 295–304. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, H.; Jiang, H.; Xu, Y.; Cao, J.; Jiang, W. Multiple 1-MCP treatment more effectively alleviated postharvest nectarine chilling injury than conventional one-time 1-MCP treatment by regulating ROS and energy metabolism. Food Chem. 2020, 330, 127256. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pei, H.; Jiao, J.; Jin, M.; Li, H.; Zhu, Q.; Ma, Y.; Rao, J. 1-Methylcyclopropene treatment followed with ethylene treatment alleviates postharvest chilling injury of ‘Xuxiang’ kiwifruit during low-temperature storage. Food Control 2021, 130, 108340. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria × anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Aghdam, M.S.; Arnao, M.B.; Brecht, J.K.; Fawole, O.A.; Pareek, S. Melatonin alleviates chilling injury symptom development in mango fruit by maintaining intracellular energy and cell wall and membrane stability. Front. Nutr. 2022, 9, 936932. [Google Scholar] [CrossRef]
- Matsumoto, S.; Obara, T.; Luh, B.S. Changes in chemical constituents of kiwifruit during post-harvest ripening. J. Food Sci. 1983, 48, 607–611. [Google Scholar] [CrossRef]
- Burdon, J.; Lallu, N.; Pidakala, P.; Barnett, A. Soluble solids accumulation and postharvest performance of ‘Hayward’ kiwifruit. Postharvest Biol. Technol. 2013, 80, 1–8. [Google Scholar] [CrossRef]
- Goldberg, T.; Agra, H.; Ben-Arie, R. Quality of ‘Hayward’ kiwifruit in prolonged cold storage as affected by the stage of maturity at Harvest. Horticulturae 2021, 7, 358. [Google Scholar] [CrossRef]
- Yaman, Ö.; Bayoιndιrlι, L. Effects of an edible coating and cold storage on shelf-life and quality of cherries. LWT-Food Sci. Technol. 2002, 35, 146–150. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Jannatizadeh, A.; Luo, Z.; Paliyath, G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Gao, S.P.; Hu, K.D.; Hu, L.Y.; Li, Y.H.; Han, Y.; Wang, H.L.; Lv, K.; Liu, Y.S.; Zhang, H. Hydrogen sulfide delays postharvest senescence and plays an antioxidative role in fresh-cut kiwifruit. HortScience 2013, 48, 1385–1392. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Jin, T.; Zhao, H.; Han, C.; Sun, F.; Chen, Q.; Yue, F.; Luo, Z.; Fu, M. Synergistic inhibitory effect of 1-methylcyclopropene (1-MCP) and chlorine dioxide (ClO2) treatment on chlorophyll degradation of green pepper fruit during storage. Postharvest Biol. Technol. 2021, 171, 111363. [Google Scholar] [CrossRef]
- Khanna-Chopra, R. Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation. Protoplasma 2012, 249, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Meng, Q.K.; Liu, X.H.; Xia, X.D.; Li, R.; Zhu, X.H.; Li, S.J. Effect of 1-MCP treatment on ascorbic acid content and its quality, physiological of Actinidia chinensis. J. Chin. Inst. Food Sci. Technol. 2014, 14, 134–141. [Google Scholar]
- Fuke, Y.; Sasago, K.; Matsuoka, H. Determination of chlorophylls in kiwi fruit and their changes during ripening. J. Food Sci. 1985, 50, 1220–1223. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, G.; Chai, J.; Yang, Y.; Ma, F.; Liu, Z. The effect of 1-MCP on the expression of carotenoid, chlorophyll degradation, and ethylene response factors in ‘Qihong’ kiwifruit. Foods 2021, 10, 3017. [Google Scholar] [CrossRef]
- Shiri, M.A.; Ghasemnezhad, M.; Moghadam, J.F.; Ebrahimi, R. Effect of CaCl2 sprays at different fruit development stages on postharvest keeping quality of “Hayward” kiwifruit. J. Food Process. Preserv. 2016, 40, 624–635. [Google Scholar] [CrossRef]
- Malekzadeh, P.; Khosravi-Nejad, F.; Hatamnia, A.A.; Mehr, R.S. Impact of postharvest exogenous γ-aminobutyric acid treatment on cucumber fruit in response to chilling tolerance. Physiol. Mol. Biol. Plants 2017, 23, 827–836. [Google Scholar] [CrossRef]
- Wang, S.Y.; Shi, X.C.; Wang, R.; Wang, H.L.; Liu, F.; Laborda, P. Melatonin in fruit production and postharvest preservation: A review. Food Chem. 2020, 320, 126642. [Google Scholar] [CrossRef] [PubMed]
- Parkin, K.L.; Marangoni, A.; Jackman, R.L.; Yada, R.Y.; Stanley, D.W. Chilling injury. A review of possible mechanisms. J. Food Biochem. 1989, 13, 127–153. [Google Scholar] [CrossRef]
- Gwanpua, S.G.; Jabbar, A.; Zhao, M.; Heyes, J.A.; East, A.R. Investigating the potential of dual temperature storage as a postharvest management practice to mitigate chilling injury in kiwifruit. Int. J. Refrig. 2018, 86, 62–72. [Google Scholar] [CrossRef]
- Azadshahraki, F.; Jamshidi, B.; Mohebbi, S. Postharvest melatonin treatment reduces chilling injury and enhances antioxidant capacity of tomato fruit during cold storage. Adv. Hortic. Sci. 2018, 32, 299–310. [Google Scholar]
- Gao, H.; Lu, Z.; Yang, Y.; Wang, D.; Yang, T.; Cao, M.; Cao, W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2018, 245, 659–666. [Google Scholar] [CrossRef]
- Jiao, W.; Xi, Y.; Cao, J.; Fan, X.; Jiang, W. Regulatory effects of CaCl2, sodium isoascorbate, and 1-methylcyclopropene on chilling injury of banana fruit at two ripening stages and the mechanisms involved. J. Food Process. Preserv. 2018, 42, e13442. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Aracil, M.C.; Guillén, F.; Ilea, M.I.M.; Martínez-Romero, D.; Lorente-Mento, J.M.; Valverde, J.M. Comparative Effect of Melatonin and 1-Methylcyclopropene Postharvest Applications for Extending ‘Hayward’ Kiwifruit Storage Life. Agriculture 2023, 13, 806. https://doi.org/10.3390/agriculture13040806
Ruiz-Aracil MC, Guillén F, Ilea MIM, Martínez-Romero D, Lorente-Mento JM, Valverde JM. Comparative Effect of Melatonin and 1-Methylcyclopropene Postharvest Applications for Extending ‘Hayward’ Kiwifruit Storage Life. Agriculture. 2023; 13(4):806. https://doi.org/10.3390/agriculture13040806
Chicago/Turabian StyleRuiz-Aracil, María Celeste, Fabián Guillén, Mihaela Iasmina Madalina Ilea, Domingo Martínez-Romero, José Manuel Lorente-Mento, and Juan Miguel Valverde. 2023. "Comparative Effect of Melatonin and 1-Methylcyclopropene Postharvest Applications for Extending ‘Hayward’ Kiwifruit Storage Life" Agriculture 13, no. 4: 806. https://doi.org/10.3390/agriculture13040806
APA StyleRuiz-Aracil, M. C., Guillén, F., Ilea, M. I. M., Martínez-Romero, D., Lorente-Mento, J. M., & Valverde, J. M. (2023). Comparative Effect of Melatonin and 1-Methylcyclopropene Postharvest Applications for Extending ‘Hayward’ Kiwifruit Storage Life. Agriculture, 13(4), 806. https://doi.org/10.3390/agriculture13040806