Non-Invasive Spectral Phenotyping Methods can Improve and Accelerate Cercospora Disease Scoring in Sugar Beet Breeding
Abstract
:1. Introduction
| Index | Formula | Indicator | Reference |
|---|---|---|---|
| NDVI, normalized difference vegetation index | (R800 - R670) × (R800 + R670)-1 | Biomass, leaf area | [14] |
| PRI, photochemical reflectance index | (R531 - R570) × (R531 + R570)-1 | Estimates xanthophyll epoxidation as a measure of photosynthetic activity | [15] |
| SIPI, structure insensitive pigment index | (R800 - R455) × (R800 + R680)-1 | Carotenoid/Chlorophyll a ratio | [16] |
| PSSRa, pigment specific simple ratio | R800 × R680-1 | Chlorophyll a | [17] |
| PSSRb | R800 × R635-1 | Chlorophyll b | [17] |
| WI, water index | R900 × R970-1 | Water content | [18] |
| CRI1, carotenoids reflectance index | R510-1 + R550-1 | Carotenoid content | [19] |
| ARI1, anthocyanin reflectance index | R550-1 + R570-1 | Anthocyanin content | [20] |
| PSNDa, pigment specific normalized difference | (R800 - R680) × (R800 + R680)-1 | Chlorophyll a | [17] |
| NDWI1240, normalized difference water index | (R980 - R1240) × (R980 + R1240)-1 | Water content | [21] |
| NDWI1640 | (R858 - R1640) × (R858 + R1640)-1 | Water content | [22] |
| LWI, leaf water index | R1300 × R1450-1 | Water content | [23] |
| CLSI, Cercospora leaf spot index | (R698 - R570) × (R698 + R570)-1 + R734 | Cercospora leaf spot classification | [13] |
2. Results and Discussion

2.1. Tetracam Allowed Quantitative Spectral Imaging of Diseased Vegetation
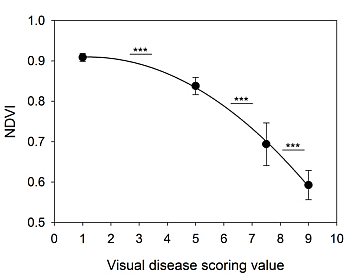
2.2. FieldSpec Hyperspectral Data Enabled Assessment of Multiple Vegetation Indices



| Index | Sum of Squares | Mean of Squares | F Value | p |
|---|---|---|---|---|
| NDVI | 0.73 | 0.36 | 111.76 | 0.00 |
| PRI | 0.01 | 0.01 | 49.27 | 4.63 × 10−12 |
| SIPI | 0.30 | 0.15 | 127.24 | 0.00 |
| PSSRa | 271.85 | 135.93 | 145.48 | 0.00 |
| PSSRb | 63.56 | 31.78 | 115.59 | 0.00 |
| WI | 1.30 | 0.65 | 110.08 | 0.00 |
| CRI1 | 0.00 | 0.00 | 5.55 | 0.01 |
| ARI1 | 0.00 | 0.00 | 7.35 | 0.00 |
| PSNDa | 0.78 | 0.39 | 112.78 | 0.00 |
| NDWI1240 | 0.78 | 0.39 | 112.78 | 0.00 |
| NDWI1640 | 1.03 | 0.51 | 124.22 | 0.00 |
| LWI | 5688.85 | 2844.43 | 69.73 | 1.64 × 10−14 |
| CLSI | 5.50 × 107 | 2.75 × 107 | 33.85 | 1.07 × 10−9 |
3. Experimental Section
4. Conclusions
Acknowledgments
Authors Contribution
Conflicts of Interest
References
- Shane, W.W.; Teng, P.S. Impact of Cercospora leaf spot on root weight, sugar yield and purity of Beta vulgaris. Plant Dis. 1992, 76, 812–820. [Google Scholar] [CrossRef]
- Rossi, V.; Battilani, P.; Chiusa, G.; Giosue, S.; Languasco, L.; Racca, P. Components of rate-reducing resistance to Cercospora leaf spot in sugar beet: Conidiation length, spore yield. J. Plant Pathol. 2000, 82, 125–131. [Google Scholar]
- Smith, G.A.; Campbell, L.G. Association between resistance to Cercospora and yield in commercial sugarbeet hybrids. Plant Breed. 1996, 115, 28–32. [Google Scholar] [CrossRef]
- Jansen, M.; Pinto, F.; Nagel, K.A.; Dusschoten, D.; Fiorani, F.; Rascher, U.; Schneider, H.U.; Walter, A.; Schurr, U. Non-invasive phenotyping methodologies enable the accurate characterization of growth and performance of shoots and roots. In Genomics of Plant Genetic Resources; Tuberosa, R., Graner, A., Frison, E., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 173–206. [Google Scholar]
- Rascher, U.; Blossfeld, S.; Fiorani, F.; Jahnke, S.; Jansen, M.; Kuhn, A.J.; Matsubara, S.; Martin, L.L.A.; Merchant, A.; Metzner, R.; et al. Non-invasive approaches for phenotyping of enhanced performance traits in bean. Funct. Plant Biol. 2011, 38, 968–983. [Google Scholar] [CrossRef]
- Hillnhütter, C.; Mahlein, A.-K. Neue ansätze zur frühzeitigen erkennung und lokalisierung von zuckerrübenkrankheiten. Gesunde Pflanzen 2008, 60, 143–149. [Google Scholar] [CrossRef]
- West, J.S.; Bravo, C.; Oberti, R.; Lemaire, D.; Moshou, D.; McCartney, H.A. The potential of optical canopy measurement for targeted control of field crop diseases. Annu. Rev. Phytopathol. 2003, 41, 593–614. [Google Scholar] [CrossRef]
- Rascher, U.; Agati, G.; Alonso, L.; Cecchi, G.; Champagne, S.; Colombo, R.; Damm, A.; Daumard, F.; de Miguel, E.; Fernandez, G.; et al. Cefles2: The remote sensing component to quantify photosynthetic efficiency from the leaf to the region by measuring sun-induced fluorescence in the oxygen absorption bands. Biogeosciences 2009, 6, 1181–1198. [Google Scholar] [CrossRef] [Green Version]
- Buschmann, C.; Nagel, E. In vivo spectroscopy and internal optics of leaves as basis for remote-sensing of vegetation. Int. J. Remote Sens. 1993, 14, 711–722. [Google Scholar] [CrossRef]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar]
- Bock, C.H.; Poole, G.H.; Parker, P.E.; Gottwald, T.R. Plant disease severity estimated visually, by digital photography and image analysis, and by hyperspectral imaging. Crit. Rev. Plant Sci. 2010, 29, 59–107. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Mahlein, A.K.; Rumpf, T.; Welke, P.; Dehne, H.W.; Plümer, L.; Steiner, U.; Oerke, E.C. Development of spectral indices for detecting and identifying plant diseases. Remote Sens. Environ. 2013, 128, 21–30. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the great plains with ERTS. In Proceedings of the Third ERTS Symposium; NASA SP-351. NASA: Washington, DC, USA, 1973; pp. 309–317. [Google Scholar]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Penuelas, J.; Baret, F.; Filella, I. Semiempirical indexes to assess carotenoids chlorophyll-a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Blackburn, G.A. Spectral indices for estimating photosynthetic pigment concentrations: A test using senescent tree leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Peñuelas, J.; Piñol, J.; Ogaya, R.; Filella, I. Estimation of plant water concentration by the reflectance water index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar]
- Gao, B.-C. Ndwi—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Chen, D.; Huang, J.; Jackson, T.J. Vegetation water content estimation for corn and soybeans using spectral indices derived from modis near- and short-wave infrared bands. Remote Sens. Environ. 2005, 98, 225–236. [Google Scholar] [CrossRef]
- Seelig, H.-D.; Hoehn, A.; Stodieck, L.S.; Klaus, D.M.; Emery, W.J.; Adams, W.W., III. Relations of remote sensing leaf water indices to leaf water thickness in cowpea, bean, and sugarbeet plants. Remote Sens. Environ. 2008, 112, 445–455. [Google Scholar] [CrossRef]
- Weiland, J.; Koch, G. Sugarbeet leaf spot disease (Cercospora beticola Sacc.). Mol. Plant Pathol. 2004, 5, 157–166. [Google Scholar] [CrossRef]
- Rumpf, T.; Mahlein, A.K.; Steiner, U.; Oerke, E.C.; Dehne, H.W.; Pluemer, L. Early detection and classification of plant diseases with support vector machines based on hyperspectral reflectance. Comput. Electron. Agric. 2010, 74, 91–99. [Google Scholar]
- Mahlein, A.K.; Steiner, U.; Dehne, H.W.; Oerke, E.C. Spectral signatures of sugar beet leaves for the detection and differentiation of diseases. Precis. Agric. 2010, 11, 413–431. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jansen, M.; Bergsträsser, S.; Schmittgen, S.; Müller-Linow, M.; Rascher, U. Non-Invasive Spectral Phenotyping Methods can Improve and Accelerate Cercospora Disease Scoring in Sugar Beet Breeding. Agriculture 2014, 4, 147-158. https://doi.org/10.3390/agriculture4020147
Jansen M, Bergsträsser S, Schmittgen S, Müller-Linow M, Rascher U. Non-Invasive Spectral Phenotyping Methods can Improve and Accelerate Cercospora Disease Scoring in Sugar Beet Breeding. Agriculture. 2014; 4(2):147-158. https://doi.org/10.3390/agriculture4020147
Chicago/Turabian StyleJansen, Marcus, Sergej Bergsträsser, Simone Schmittgen, Mark Müller-Linow, and Uwe Rascher. 2014. "Non-Invasive Spectral Phenotyping Methods can Improve and Accelerate Cercospora Disease Scoring in Sugar Beet Breeding" Agriculture 4, no. 2: 147-158. https://doi.org/10.3390/agriculture4020147
APA StyleJansen, M., Bergsträsser, S., Schmittgen, S., Müller-Linow, M., & Rascher, U. (2014). Non-Invasive Spectral Phenotyping Methods can Improve and Accelerate Cercospora Disease Scoring in Sugar Beet Breeding. Agriculture, 4(2), 147-158. https://doi.org/10.3390/agriculture4020147