Aflatoxins in Mozambique: Impact and Potential for Intervention
Abstract
:1. Introduction
2. Impact
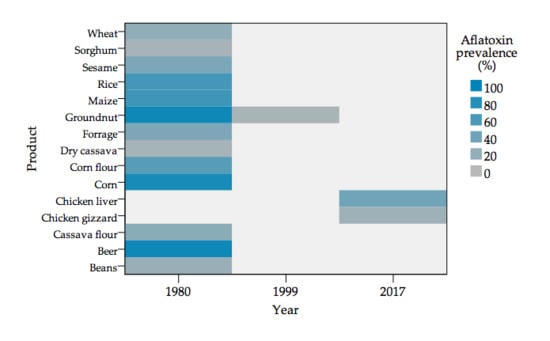
2.1. Risk Analysis
2.2. Human and Animal Health
2.3. Etiology
3. Resources
3.1. Facilities
- National Laboratory of Water and Food Hygiene (LNHAA);
- Directorate of Animal Sciences. The Mozambican Institute for Research on Agriculture (IIAM).
3.2. Projects
3.3. Personnel
4. Levels and Regulation
5. Conclusions
Funding
Conflicts of Interest
References
- Stevens, A.; Saunders, C.; Spence, J.; Newham, A. Investigations into “diseases” of turkey poults. Vet. Rec. 1960, 72, 627–628. [Google Scholar]
- Van Der Zijden, A.S.M.; Koelensmid, W.A.A.B.; Boldingh, J.; Barrett, C.B.; Ord, W.O.; Philp, J. Aspergillus flavus and turkey X disease: Isolation in crystalline form of a toxin responsible for turkey X disease. Nature 1962, 195, 1060–1062. [Google Scholar] [CrossRef]
- Krishnamachari, K.; Nagarajan, V.; Bhat, R.; Tilak, T. Hepatitis due to aflatoxicosis: An outbreak in western india. Lancet 1975, 305, 1061–1063. [Google Scholar] [CrossRef]
- Lewis, L.; Onsongo, M.; Njapau, H.; Schurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central kenya. Environ. Health Perspect. 2005, 113, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Van Rensburg, S.J.; Cook-Mozaffari, P.; Van Schalkwyk, D.J.; Van der Watt, J.J.; Vincent, T.J.; Purchase, I.F. Hepatocellular carcinoma and dietary aflatoxin in mozambique and transkei. Br. J. Cancer 1985, 51, 713–726. [Google Scholar] [CrossRef] [PubMed]
- SN. Redução de Perdas Pós-Colheitas: Moçambique Passa a Produzir Biocontrolos. Available online: http://www.jornalnoticias.co.mz/index.php/ciencia-e-ambiente/2466-reducao-de-perdas-pos-colheitas-mocambique-passa-a-produzir-biocontrolos.html (accessed on 20 May 2018).
- Van Wyk, P.; Van der Merwe, P.; Subrahmanyam, P.; Boughton, D. Aflatoxin contamination of groundnuts in mozambique. Int. Arachis Newsl. 1999, 19, 25–27. [Google Scholar]
- Sineque, A.R.; Macuamule, C.L.; Dos Anjos, F.R. Aflatoxin b1 contamination in chicken livers and gizzards from industrial and small abattoirs, measured by elisa technique in maputo, mozambique. Int. J. Environ. Res. Public Health 2017, 14, 951. [Google Scholar] [CrossRef] [PubMed]
- Van Rensburg, S.J.; Kirsipuu, A.; Coutinho, L.P.; Van Der Watt, J.J. Circumstances associated with the contamination of food by aflatoxin in a high primary liver cancer area. S. Afr. Med. J. 1975, 49, 877–883. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention. Outbreak of aflatoxin poisoning—Eastern and central provinces, Kenya, January–July 2004. MMWR. Morb. Mortal. Wkly. Rep. 2004, 53, 790–793. [Google Scholar]
- Linsell, C.A.; Peers, F.G. Aflatoxin and liver cell cancer. Trans. R. Soc. Trop. Med. Hyg. 1977, 71, 471–473. [Google Scholar] [CrossRef]
- Casadei, E. Os contaminantes nos alimentos. In Mocambique: Águas, Alimentos e Ambiente; Molisv: Rome, Italy, 1980. [Google Scholar]
- Warth, B.; Parich, A.; Atehnkeng, J.; Bandyopadhyay, R.; Schuhmacher, R.; Sulyok, M.; Krska, R. Quantitation of mycotoxins in food and feed from burkina faso and mozambique using a modern lc-ms/ms multitoxin method. J. Agric. Food Chem. 2012, 60, 9352–9363. [Google Scholar] [CrossRef] [PubMed]
- Griggs, D.; Stafford-Smith, M.; Gaffney, O.; Rockström, J.; Öhman, M.C.; Shyamsundar, P.; Steffen, W.; Glaser, G.; Kanie, N.; Noble, I. Policy: Sustainable development goals for people and planet. Nature 2013, 495, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Cabinet Council. Plano de Acção para a Redução da Pobreza Absoluta; Conselho de Ministros: Maputo, Mozambique, 2006. [Google Scholar]
- Codex Alimentarius Commission. Codex General Standard for Contaminants and Toxins in Food and Feed; Codex stan 193-1995; Codex Alimentarius Commission: Washington, DC, USA, 2013. [Google Scholar]
- Augusto, J.; Atehnkeng, J.; Akello, J.; Cotty, P.; Bandyopadhyay, R. Prevalence and distribution of aspergillus section flavi in maize and groundnut fields and aflatoxin contamination in mozambique. In 2014 APS-CPS Joint Meeting; The American Phytopathological Society: Minneapolis, MN, USA, 2014. [Google Scholar]
- Harmsen, J.; Bremmer, J.; Maria, R.M. Pre Evaluation of a Soil and Plant Laboratory in Mozambique; Alterra: Wageningen, The Netherlands, 2012; p. 60. [Google Scholar]
- Anjos, F.D.; Ledoux, D.; Rottinghaus, G.; Chimonyo, M. Efficacy of mozambican bentonite and diatomaceous earth in reducing the toxic effects of aflatoxins in chicks. World Mycotoxin J. 2016, 9, 63–72. [Google Scholar] [CrossRef]
- PACA. What Is the Aflatoxin Problem? Available online: http://aflatoxinpartnership.org/?q=node/413 (accessed on 2 May 2018).
- Ozturk, M. P53 mutation in hepatocellular carcinoma after aflatoxin exposure. Lancet 1991, 338, 1356–1359. [Google Scholar] [PubMed]
- Muitia, A. Farmer Perceptions and Genetic Studies of Rosette Disease in Groundnut (Arachis hypogaea L.) in Northern Mozambique. Ph.D. Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2011. [Google Scholar]
- Rahmianna, A.; Taufiq, A.; Yusnawan, E. Effect of harvest timing and postharvest storage conditions on aflatoxin contamination in groundnuts harvested from the wonogiri regency in indonesia. ICRISAT 2007, 5, 1–3. [Google Scholar]
- Muitia, A.M. Combination of Root-Knot Nematode (Meloidogyne spp.) Resistance and Edible Seed Quality for Peanut (Arachis hypogaea L.) Production in Mozambique and in the US. Ph.D. Thesis, Texas Tech University, Lubbock, TX, USA, 2005. [Google Scholar]
- Zuza, E.; Mondjana, A.; Muitia, A.; Amane, M. Effects of harvesting date on aflatoxin contamination in groundnuts in northern mozambique. In The Fifth RUFORUM Biennial Conference and African Higher Education Week; Nampala, M.P., Egeru, A., Tusiime, G., Osiru, M., Mensah, S., Adipala, E., Eds.; Regional Universities Forum for Capacity Building in Agriculture (RUFORUM): Cape Town, South Africa, 2016; Volume 14, pp. 167–172. [Google Scholar]
- Arias, F.J.; Libombo, M. Groundnut Evaluation in Mozambique: Preliminary Results for the 1993/94 Season in Maputo Province; Ndunguru, B.J., Hildebrand, G.L., Subrahmanyam, P., Eds.; Sustainable Groundnut Production in Southern and Eastern Africa, Mbabane, 1994; International Crops Research Institute for the Semi-Arid Tropics: Mbabane, Swaziland, 1994. [Google Scholar]
- Walker, T.; Pitoro, R.; Tomo, A.; Sitoe, I.; Salência, C.; Mahanzule, R.; Donovan, C.; Mazuze, F. Priority Setting for Public-Sector Agricultural Research in Mozambique with the National Agricultural Survey Data; Directorate of Training, Documentation, and Technology Transfer, Institute of Agricultural Research of Mozambique: Maputo, Mozambique, 2006; p. 80. [Google Scholar]
- Bandyopadhyay, R.; Dubois, T. Aflatoxin control projects launched in Southern Africa. IIAPPS Newsl. 2012, 2, 2–4. [Google Scholar]
- ALM. Directório dos Laboratórios de Calibração e de Ensaios em Moçambique; Associação de Laboratórios de Moçambique: Vienna, Austria, 2013; p. 69. [Google Scholar]
- Mondlane, I.A.P.; Capece, B.P.S.; Parruque, A.F. Relação entre a Ocorrência de Fungos e a Presença de Aflatoxinas b1 em Rações para Aves Fabricadas em Maputo; Instituto de Investigação Agrária de Moçambique: Maputo, Mozambique, 2005. [Google Scholar]
- Baquete, E.; Freire, M. Present status and perspectives of aflatoxin research in mozambique. Aflatoxin Contam. Groundn. 1989, 93–94. Available online: http://oar.icrisat.org/502/1/RA_00132.pdf#page=263 (accessed on 10 May 2005).
- U.S. Embassy Maputo. Inauguration of Iita’s New Facilities in the Celebration of Its 50th Year Anniversary. Available online: https://mz.usembassy.gov/inauguration-iitas-new-facilities-celebration-50th-year-anniversary/ (accessed on 10 May 2018).
- UCM. Licenciatura em Engenharia Alimentar. Available online: http://www.ucm.ac.mz/cms/node/254 (accessed on 3 May 2018).
- UEM. Uem Introduz Licenciatura em Tecnologia de Alimentos. Available online: http://www.uem.mz/index.php/noticias-recentes/380-uem-introduz-licenciatura-em-tecnologia-de-alimentos (accessed on 3 May 2018).
- ISPG. Plano Curricular do Curso de Licenciatura em Engenharia de Processamento de Alimentos; Instituto Superior Politécnico de Gaza: Lionde, Mozambique, 2016. [Google Scholar]
- Wu, F.; Guclu, H. Aflatoxin regulations in a network of global maize trade. PLoS ONE 2012, 7, e45151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Stacy, S.L.; Kensler, T.W. Global risk assessment of aflatoxins in maize and peanuts: Are regulatory standards adequately protective? Toxicol. Sci. 2013, 135, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Akrobortu, D.E. Aflatoxin Contamination of Maize from Different Storage Locations in Ghana. Ph.D. Thesis, Kwame Nkrumah University of Science and Technology, Accra, Ghana, 2008. [Google Scholar]
- Government of Mozambique. Regulamento da lei de Defesa do Consumidor; In 7; Assembleia da República, Ed.; Imprensa Nacional de Moçambique: Maputo, Mozambique, 2016; Volume 27, pp. 505–510.
- Carlson, M.P.; Ensley, S.M. Sampling and Analyzing Feed for Fungal (Mold) Toxins (Mycotoxins); Cooperative Extension, Institute of Agriculture and Natural Resources, University of Nebraska-Lincoln: Lincoln, NE, USA, 2003. [Google Scholar]
- Di Stefano, V.; Pitonzo, R.; Cicero, N.; D’Oca, M.C. Mycotoxin contamination of animal feedingstuff: Detoxification by gamma-irradiation and reduction of aflatoxins and ochratoxin a concentrations. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 2034–2039. [Google Scholar] [CrossRef] [PubMed]





| Province | District/City | Codex Evaluation (%) | Total (%) | Products | |
|---|---|---|---|---|---|
| Acceptable | Unacceptable | ||||
| Maputo | Maputo | 100 | 0 | 100 | Chicken liver and gizzard |
| Inhambane | Inhambane | 50 | 50 | 100 | Groundnut Maize |
| Nampula | Amendo | 0 | 100 | 100 | Groundnut Maize Millet Feed waste |
| Erati | 50 | 50 | 100 | ||
| Maletna | 100 | 0 | 100 | ||
| Mecuburi | 100 | 0 | 100 | ||
| Muabassa | 100 | 0 | 100 | ||
| Muecate | 0 | 100 | 100 | ||
| Mugovola | 33.3 | 66.7 | 100 | ||
| Murrupula | 50 | 50 | 100 | ||
| Nacala | 100 | 0 | 100 | ||
| Nacaroa | 100 | 0 | 100 | ||
| Nampula | 60 | 40 | 100 | ||
| Ribaue | 100 | 0 | 100 | ||
| Overall | 63.2 | 36.8 | 100 | ||
| Product | Codex Evaluation (%) | Total (%) | |
|---|---|---|---|
| Acceptable | Unacceptable | ||
| Beans | 100 | 0 | 100 |
| Beer | 100 | 0 | 100 |
| Cassava flour | 0 | 100 | 100 |
| Chicken gizzard | 100 | 0 | 100 |
| Chicken liver | 100 | 0 | 100 |
| Corn | 0 | 100 | 100 |
| Corn flour | 100 | 0 | 100 |
| Dry cassava | 100 | 0 | 100 |
| Feed waste | 0 | 100 | 100 |
| Forage | 0 | 100 | 100 |
| Groundnut | 60 | 40 | 100 |
| Maize | 33.3 | 66.7 | 100 |
| Millet | 100 | 0 | 100 |
| Rice | 100 | 0 | 100 |
| Sesame | 100 | 0 | 100 |
| Sorghum | 100 | 0 | 100 |
| Wheat | 100 | 0 | 100 |
| Overall | 63.2 | 36.8 | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cambaza, E.; Koseki, S.; Kawamura, S. Aflatoxins in Mozambique: Impact and Potential for Intervention. Agriculture 2018, 8, 100. https://doi.org/10.3390/agriculture8070100
Cambaza E, Koseki S, Kawamura S. Aflatoxins in Mozambique: Impact and Potential for Intervention. Agriculture. 2018; 8(7):100. https://doi.org/10.3390/agriculture8070100
Chicago/Turabian StyleCambaza, Edgar, Shigenobu Koseki, and Shuso Kawamura. 2018. "Aflatoxins in Mozambique: Impact and Potential for Intervention" Agriculture 8, no. 7: 100. https://doi.org/10.3390/agriculture8070100
APA StyleCambaza, E., Koseki, S., & Kawamura, S. (2018). Aflatoxins in Mozambique: Impact and Potential for Intervention. Agriculture, 8(7), 100. https://doi.org/10.3390/agriculture8070100