Fabrication of Fluorine-Free Superhydrophobic Surface on Aluminum Substrate for Corrosion Protection and Drag Reduction
Abstract
:1. Introduction
2. Materials and Methods
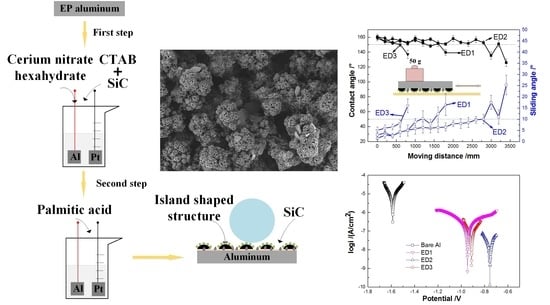
2.1. Preparation of Fluorine-Free Superhydrophobic Surfaces
2.2. Characterizations and Performance Tests
3. Results
3.1. Microstructure Morphology
3.2. Water Contact Angles
3.3. Chemical Composition
3.4. Electrodeposition Process
3.5. Mechanical Durability Performance
3.6. Anti-Corrosion Performance
3.7. Liquid Impalement Resistance Property
3.8. Drag Reduction Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, X.; Hu, J.; Ruan, T.; Zhou, J.; Qu, J. Chitosan derivative corrosion inhibitor for aluminum alloy in sodium chloride solution: A green organic/inorganic hybrid. Carbohydr. Polym. 2021, 265, 118074. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, F.C.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Protective PMMA-silica coatings for aluminum alloys: Nanostructural control of elevated thermal stability and anticorrosive performance. Prog. Org. Coat. 2021, 152, 106129. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Chen, T.; Lang, J.; Li, C.; Yan, T.; Dai, J. One-step facile fabrication of sustainable cellulose membrane with su-perhydrophobicity via a sol-gel strategy for efficient oil/water separation. Surf. Coat. Technol. 2019, 361, 19–26. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, L.; Liu, X.; Weng, R.; Wang, Y.; Wu, Z. Wetting behavior and drag reduction of superhydrophobic layered double hydroxides films on aluminum. Appl. Surf. Sci. 2016, 380, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Tuo, Y.; Chen, W.; Zhang, H.; Li, P.; Liu, X. One-step hydrothermal method to fabricate drag reduction superhydrophobic surface on aluminum foil. Appl. Surf. Sci. 2018, 446, 230–235. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, S.; Dong, H.; Han, S.; Wei, H.; Shi, F. Improving the Durability of a Drag-Reducing Nanocoating by Enhancing Its Mechanical Stability. ACS Appl. Mater. Interfaces 2015, 7, 4275–4282. [Google Scholar] [CrossRef]
- Chi, Y.; Gu, G.; Yu, H.; Chen, C. Laser surface alloying on aluminum and its alloys: A review. Opt. Lasers Eng. 2018, 100, 23–37. [Google Scholar] [CrossRef]
- Zhu, J. A novel fabrication of superhydrophobic surfaces on aluminum substrate. Appl. Surf. Sci. 2018, 447, 363–367. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Zhu, Q.; Wang, X.; Zhao, X.; Sun, C.; Li, Y.; Hou, B. Controllable Dianthus caryophyllus-like superhydro-philic/superhydrophobic hierarchical structure based on self-congregated nanowires for corrosion inhibition and biofouling mitigation. Chem. Eng. J. 2017, 312, 317–327. [Google Scholar] [CrossRef]
- Kumar, A.; Gogoi, B. Development of durable self-cleaning superhydrophobic coatings for aluminium surfaces via chemical etching method. Tribol. Int. 2018, 122, 114–118. [Google Scholar] [CrossRef]
- Zhang, B.; Guan, F.; Zhao, X.; Zhang, Y.; Li, Y.; Duan, J.; Hou, B. Micro-nano textured superhydrophobic 5083 aluminum alloy as a barrier against marine corrosion and sulfate-reducing bacteria adhesion. J. Taiwan Inst. Chem. Eng. 2019, 97, 433–440. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, J.; Li, Z.; Qin, N.; Mo, J.; Pan, Y.; Luo, D. Robust superhydrophobic aluminum alloy surfaces with anti-icing ability, thermostability, and mechanical durability. Prog. Org. Coatings 2020, 147, 105745. [Google Scholar] [CrossRef]
- Fu, J.; Sun, Y.; Ji, Y.; Zhang, J. Fabrication of robust ceramic based superhydrophobic coating on aluminum substrate via plasma electrolytic oxidation and chemical vapor deposition methods. J. Mater. Process. Technol. 2022, 306, 117641. [Google Scholar] [CrossRef]
- Wang, H.; Di, D.; Zhao, Y.; Yuan, R.; Zhu, Y. A multifunctional polymer composite coating assisted with pore-forming agent: Preparation, superhydrophobicity and corrosion resistance. Prog. Org. Coatings 2019, 132, 370–378. [Google Scholar] [CrossRef]
- Li, Z.; Cao, M.; Li, P.; Zhao, Y.; Bai, H.; Wu, Y.; Jiang, L. Surface-Embedding of Functional Micro-/Nanoparticles for Achieving Versatile Superhydrophobic Interfaces. Matter 2019, 1, 661–673. [Google Scholar] [CrossRef] [Green Version]
- Rasitha, T.; Vanithakumari, S.; Krishna, D.N.G.; George, R.; Srinivasan, R.; Philip, J. Facile fabrication of robust superhydrophobic aluminum surfaces with enhanced corrosion protection and antifouling properties. Prog. Org. Coatings 2021, 162, 106560. [Google Scholar] [CrossRef]
- ECHA, European Chemical Agency. Candidate List of Substances of Very High Concern for Authorisation. 2013. Available online: https://echa.europa.eu/web/guest/candidate-list-table (accessed on 14 August 2022).
- Liu, Y.; Xue, J.; Luo, D.; Wang, H.; Gong, X.; Han, Z.; Ren, L. One-step fabrication of biomimetic superhydrophobic surface by electrodeposition on magnesium alloy and its corrosion inhibition. J. Colloid Interface Sci. 2017, 491, 313–320. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Zhao, X.; Hu, X.; Yang, L.; Wang, N.; Li, Y.; Hou, B. Biomimetic one-step fabrication of manganese stearate su-perhydrophobic surface as an efficient barrier against marine corrosion and Chlorella vulgaris-induced biofouling. Chem. Eng. J. 2016, 306, 441–451. [Google Scholar] [CrossRef]
- Fu, J.; Li, M.; Liu, G.; Ma, S.; Zhu, X.; Ma, C.; Cheng, D.; Yan, Z. Robust ceramic based self-lubricating coating on Al–Si alloys prepared via PEO and spin-coating methods. Wear 2020, 458–459, 203405. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Huang, Y.; Yang, L.; Ye, Y.; Walsh, F.C.; Chen, J.; Wang, S. Robust Ni/WC superhydrophobic surfaces by electro-deposition. RSC Adv. 2017, 7, 44896–44903. [Google Scholar] [CrossRef] [Green Version]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Bianconi, A.; Marcelli, A.; Tomellini, M.; Davoli, I. Determination of mixing of 4f-ligand orbitals in Ce(SO4)2 by Xanes is Ce(SO4)2 a mixed valent insulating system? J. Magn. Magn. Mater. 1985, 47–48, 209–211. [Google Scholar] [CrossRef]
- Sharma, S.; Hegde, M.S. Pt metal-CeO2 interaction: Direct observation of redox coupling between Pt0/Pt2+/Pt4+ and Ce4+/Ce3+ states in Ce0.98Pt0.02O2-δ catalyst by a combined electrochemical and x-ray photoelectron spectroscopy study. J. Chem. Phys. 2009, 130, 114706. [Google Scholar] [CrossRef] [PubMed]
- Sudarsanam, P.; Selvakannan, P.R.; Soni, S.K.; Bhargava, S.K.; Reddy, B.M. Structural evaluation and catalytic performance of nano-Au supported on nanocrystalline Ce0.9Fe0.1O2-δ solid solution for oxidation of carbon monoxide and benzylamine. RSC Adv. 2014, 4, 43460–43469. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, X.; Li, Y.; Hou, B. Fabrication of durable anticorrosion superhydrophobic surfaces on aluminum substrates via a facile one-step electrodeposition approach. RSC Adv. 2016, 6, 35455–35465. [Google Scholar] [CrossRef]
- Sawisai, R.; Wanchanthuek, R.; Radchatawedchakoon, W.; Sakee, U. Simple continuous flow synthesis of linoleic and palmitic acid-coated magnetite nanoparticles. Surf. Interfaces 2019, 17, 100344. [Google Scholar] [CrossRef]
- Lv, J.; Guo, S.; Li, G. In situ Raman Spectroscopic of cerium surface oxidation and reduction processes. J. Chin. Soc. Rare Earths 2013, 31, 488–494. [Google Scholar]
- Han, R.; Han, B.; Wang, D.H.; Li, C. Temperature dependence of Raman scattering from 4H-SiC with hexagonal defects. Appl. Phys. Lett. 2011, 99, 011912. [Google Scholar] [CrossRef]
- Yan, C.; Yi, W.; Ma, P.; Deng, X.; Song, S.; Zhu, Z. Synthesis and Characterization of Hydrous Ceria with its adsorption property for Boron. J. Chin. Soc. Rare Earths 2008, 26, 5. [Google Scholar]
- Nine, J.; Cole, M.A.; Johnson, L.; Tran, D.N.H.; Losic, D. Robust Superhydrophobic Graphene-Based Composite Coatings with Self-Cleaning and Corrosion Barrier Properties. ACS Appl. Mater. Interfaces 2015, 7, 28482–28493. [Google Scholar] [CrossRef]
- Lambley, H.; Schutzius, T.M.; Poulikakos, D. Superhydrophobic surfaces for extreme environmental conditions. Proc. Natl. Acad. Sci. USA 2020, 117, 27188–27194. [Google Scholar] [CrossRef]









| Samples | Ecorr (V) | icorr (Acm−2) |
|---|---|---|
| ED1 | −0.946 | 2.924 × 10−8 |
| ED2 | −0.755 | 5.224 × 10−9 |
| ED3 | −0.917 | 1.799 × 10−8 |
| Al substrate | −1.587 | 1.159 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Sun, Y.; Wang, J.; Zhang, H.; Zhang, J.; Ji, Y. Fabrication of Fluorine-Free Superhydrophobic Surface on Aluminum Substrate for Corrosion Protection and Drag Reduction. J. Mar. Sci. Eng. 2023, 11, 520. https://doi.org/10.3390/jmse11030520
Fu J, Sun Y, Wang J, Zhang H, Zhang J, Ji Y. Fabrication of Fluorine-Free Superhydrophobic Surface on Aluminum Substrate for Corrosion Protection and Drag Reduction. Journal of Marine Science and Engineering. 2023; 11(3):520. https://doi.org/10.3390/jmse11030520
Chicago/Turabian StyleFu, Jingguo, Yihe Sun, Jingye Wang, Hongpeng Zhang, Jifeng Zhang, and Yulong Ji. 2023. "Fabrication of Fluorine-Free Superhydrophobic Surface on Aluminum Substrate for Corrosion Protection and Drag Reduction" Journal of Marine Science and Engineering 11, no. 3: 520. https://doi.org/10.3390/jmse11030520
APA StyleFu, J., Sun, Y., Wang, J., Zhang, H., Zhang, J., & Ji, Y. (2023). Fabrication of Fluorine-Free Superhydrophobic Surface on Aluminum Substrate for Corrosion Protection and Drag Reduction. Journal of Marine Science and Engineering, 11(3), 520. https://doi.org/10.3390/jmse11030520