Thermal Shock and Ciprofloxacin Act Orthogonally on Pseudomonas aeruginosa Biofilms
Abstract
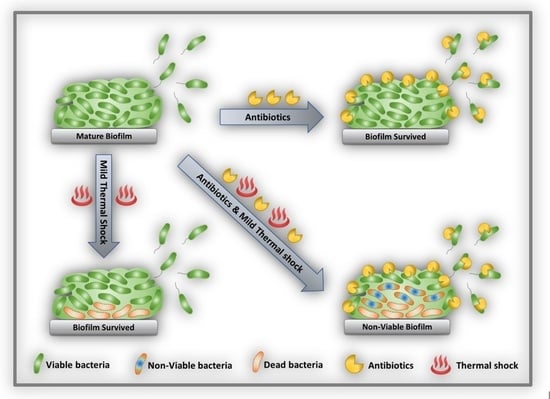
:1. Introduction
2. Results
2.1. Population Density, Architecture, and Thermal Susceptibility
2.2. Re-Incubation
2.3. Antibiotic Exposure
2.4. Combined Antibiotic and Thermal Exposure of Shaker Table (ST) Biofilms
2.5. Combined Antibiotic and Thermal Exposure of Drip Flow Reactor (DFR) Biofilms
3. Discussion
4. Materials and Methods
4.1. Streak and Inoculum
4.2. Biofilm Culture
4.2.1. Shaker Table Biofilm
4.2.2. Drip Flow Reactor (DFR) Biofilm
4.3. Thermal Shock
4.4. Re-Incubation
4.5. Antibiotic Exposure
4.5.1. Shaker Table Antibiotic Exposure
4.5.2. Drip Flow Reactor Antibiotic Exposure
4.6. Antibiotic and Thermal Exposure
4.7. Enumeration
4.8. Confocal Microscopy
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDermott, K.W.; Freeman, W.J.; Elixhauser, A. Overview of Operating Room Procedures during Inpatient Stays in U.S. Hospitals, 2014; HCUP Statistical brief #233; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2017. [Google Scholar]
- O’Toole, P.; Maltenfort, M.G.; Chen, A.F.; Parvizi, J. Projected increase in periprosthetic joint infections secondary to rise in diabetes and obesity. J. Arthroplast. 2016, 31, 7–10. [Google Scholar] [CrossRef]
- Parvizi, J.; Ghanem, E.; Azzam, K.; Davis, E.; Jaberi, F.; Hozack, W. Periprosthetic infection: Are current treatment strategies adequate? Acta Orthop. Belg. 2008, 74, 793–800. [Google Scholar]
- Phillips, J.E.; Crane, T.P.; Noy, M.; Elliott, T.S.J.; Grimer, R.J. The incidence of deep prosthetic infections in a specialist orthopaedic hospital. J. Bone Jt. Surg. Br. Vol. 2006, 88, 943–948. [Google Scholar] [CrossRef]
- Cui, Q.; Mihalko, W.M.; Shields, J.S.; Ries, M.; Saleh, K.J. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J. Bone Jt. Surg. Am. Vol. 2007, 89, 871–882. [Google Scholar] [CrossRef]
- Anderl, J.N.; Zahller, J.; Roe, F.; Stewart, P. Role of nutrient limitation and stationary-phase existence in klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2003, 47, 1251–1256. [Google Scholar] [CrossRef] [Green Version]
- Piddock, L.J.V. Multidrug-resistance efflux pumps? Not just for resistance. Nat. Rev. Genet. 2006, 4, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kamesh, A.C.; Xiao, Y.; Sun, V.; Hayes, M.; Daniell, H.; Koo, H. Topical delivery of low-cost protein drug candidates made in chloroplasts for biofilm disruption and uptake by oral epithelial cells. Biomaterials 2016, 105, 156–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.; Alvarado-Gomez, E.; Magaña-Aquino, M.; Sánchez-Sánchez, R.; Velasquillo, C.; Gonzalez, C.; Ganem-Rondero, A.; Martinez-Castanon, G.-A.; Zavala-Alonso, N.; Martinez-Gutierrez, F. Anti-biofilm activity of chitosan gels formulated with silver nanoparticles and their cytotoxic effect on human fibroblasts. Mater. Sci. Eng. C 2016, 60, 317–323. [Google Scholar] [CrossRef]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef]
- Tran, N.; Tran, P. Nanomaterial-based treatments for medical device-associated infections. ChemPhysChem 2012, 13, 2481–2494. [Google Scholar] [CrossRef] [PubMed]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Executive summary: Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berend, K.R.; Lombardi, A.V.; Morris, M.J.; Bergeson, A.G.; Adams, J.; Sneller, M.A. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin. Orthop. Relat. Res. 2013, 471, 510–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, H.; Della Valle, C.J. The two-stage standard in revision total hip replacement. Bone Jt. J. 2013, 95-B, 84–87. [Google Scholar] [CrossRef]
- Rohde, H.; Burandt, E.C.; Siemssen, N.; Frommelt, L.; Burdelski, C.; Wurster, S.; Scherpe, S.; Davies, A.; Harris, L.; Horstkotte, M.A.; et al. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials 2007, 28, 1711–1720. [Google Scholar] [CrossRef]
- Wilkins, M.; Hall-Stoodley, L.; Allan, R.; Faust, S.N. New approaches to the treatment of biofilm-related infections. J. Infect. 2014, 69, S47–S52. [Google Scholar] [CrossRef]
- Tulloch, A.W.; Chun, Y.; Kealey, C.; Mohanchandra, K.P.; Chang, J.; Milisavljevic, V.; Levi, D.S.; Lawrence, P.F.; Rigberg, D.A. PS236. Hydrophilic surface treatment of thin film nickel titanium reduces bacterial biofilm production compared to commercially available endograft materials. J. Vasc. Surg. 2010, 51, 79S–80S. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Ravn, D.B.; Gram, L.; Kingshott, P. Stainless steel modified with poly(ethylene glycol) can prevent protein adsorption but not bacterial adhesion. Colloids Surf. B Biointerfaces 2003, 32, 275–291. [Google Scholar] [CrossRef]
- Tunney, M.; Gorman, S. Evaluation of a poly(vinyl pyrollidone)-coated biomaterial for urological use. Biomaterials 2002, 23, 4601–4608. [Google Scholar] [CrossRef]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Koopaie, M.; Bordbar-Khiabani, A.; Kolahdooz, S.; Darbandsari, A.K.; Mozafari, M. Advanced surface treatment techniques counteract biofilm-associated infections on dental implants. Mater. Res. Express 2020, 7, 015417. [Google Scholar] [CrossRef]
- Chen, J.; Howell, C.; Haller, C.A.; Patel, M.S.; Ayala, P.; Moravec, K.A.; Dai, E.; Liu, L.; Sotiri, I.; Aizenberg, M.; et al. An immobilized liquid interface prevents device associated bacterial infection in vivo. Biomaterials 2017, 113, 80–92. [Google Scholar] [CrossRef] [Green Version]
- Khare, M.D.; Bukhari, S.S.; Swann, A.; Spiers, P.; McLaren, I.; Myers, J. Reduction of catheter-related colonisation by the use of a silver zeolite-impregnated central vascular catheter in adult critical care. J. Infect. 2007, 54, 146–150. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.; et al. Rapid biofilm eradication on bone implants using red phosphorus and near-infrared light. Adv. Mater. 2018, 30, e1801808. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, D. Controlling Streptococcus mutans and Staphylococcus aureus biofilms with direct current and chlorhexidine. AMB Express 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Coffel, J.; Nuxoll, E. Magnetic nanoparticle/polymer composites for medical implant infection control. J. Mater. Chem. B 2015, 3, 7538–7545. [Google Scholar] [CrossRef] [PubMed]
- Van der Borden, A.; van der Mei, H.; Busscher, H. Electric block current induced detachment from surgical stainless steel and decreased viability of Staphylococcus epidermidis. Biomaterials 2005, 26, 6731–6735. [Google Scholar] [CrossRef]
- Carmen, J.C.; Roeder, B.L.; Nelson, J.L.; Ogilvie, R.L.R.; Robison, R.; Schaalje, G.B.; Pitt, W.G. Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics. Am. J. Infect. Control. 2005, 33, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Zhang, X.; Yang, Y.; Zhang, H.; Shi, J.; Yao, X. Near-infrared light-triggered therapy to combat bacterial biofilm infections by MoSe2 /TiO2 nanorod arrays on bone implants. Adv. Mater. Interfaces 2019, 7. [Google Scholar] [CrossRef]
- Richardson, I.P.; Sturtevant, R.; Heung, M.; Solomon, M.; Younger, J.G.; Vanepps, J.S. Hemodialysis catheter heat transfer for biofilm prevention and treatment. ASAIO J. 2016, 62, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Bandara, H.; Nguyen, D.; Mogarala, S.; Osiñski, M.; Smyth, H. Magnetic fields suppress Pseudomonas aeruginosa biofilms and enhance ciprofloxacin activity. Biofouling 2015, 31, 443–457. [Google Scholar] [CrossRef]
- O’Toole, A.; Ricker, E.B.; Nuxoll, E. Thermal mitigation of Pseudomonas aeruginosabiofilms. Biofouling 2015, 31, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Chopra, R.; Shaikh, S.; Chatzinoff, Y.; Munaweera, I.; Cheng, B.; Daly, S.M.; Xi, Y.; Bing, C.; Burns, D.; Greenberg, D.E. Employing high-frequency alternating magnetic fields for the non-invasive treatment of prosthetic joint infections. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricker, E.B.; Nuxoll, E. Synergistic effects of heat and antibiotics on Pseudomonas aeruginosa biofilms. Biofouling 2017, 33, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, S.; Holinka, J.; Reichmann, S.; Hirschl, A.M.; Graninger, W.; Presterl, E. Increased temperature enhances the antimicrobial effects of daptomycin, vancomycin, tigecycline, fosfomycin, and cefamandole on staphylococcal biofilms. Antimicrob. Agents Chemother. 2010, 54, 4078–4084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Wang, L.; Gong, M.; Lin, Y.; Xu, Y.; Ye, L.; Yu, X.; Liu, J.; Liu, J.; He, S.; et al. Synergistic effects of nanoparticle heating and amoxicillin on H. pylori inhibition. J. Magn. Magn. Mater. 2019, 485, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Pijls, B.G.; Sanders, I.M.J.G.; Kuijper, E.J.; Nelissen, R.G.H.H. Synergy between induction heating, antibiotics, and N-acetylcysteine eradicates Staphylococcus aureus from biofilm. Int. J. Hyperth. 2020, 37, 130–136. [Google Scholar] [CrossRef] [Green Version]
- McEvoy, G.K. (Ed.) AHFS Drug Information 2008; American Society of Health-System Pharmacists: Bethesda, MD, USA, 2008. [Google Scholar]
- Ricker, E.B.; Aljaafari, H.A.S.; Bader, T.M.; Hundley, B.S.; Nuxoll, E. Thermal shock susceptibility and regrowth of Pseudomonas aeruginosabiofilms. Int. J. Hyperth. 2018, 34, 168–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochran, W.J. Approximate significance levels of the behrens-fischer test. Biometrics 1964, 20, 191–195. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljaafari, H.; Gu, Y.; Chicchelly, H.; Nuxoll, E. Thermal Shock and Ciprofloxacin Act Orthogonally on Pseudomonas aeruginosa Biofilms. Antibiotics 2021, 10, 1017. https://doi.org/10.3390/antibiotics10081017
Aljaafari H, Gu Y, Chicchelly H, Nuxoll E. Thermal Shock and Ciprofloxacin Act Orthogonally on Pseudomonas aeruginosa Biofilms. Antibiotics. 2021; 10(8):1017. https://doi.org/10.3390/antibiotics10081017
Chicago/Turabian StyleAljaafari, Haydar, Yuejia Gu, Hannah Chicchelly, and Eric Nuxoll. 2021. "Thermal Shock and Ciprofloxacin Act Orthogonally on Pseudomonas aeruginosa Biofilms" Antibiotics 10, no. 8: 1017. https://doi.org/10.3390/antibiotics10081017
APA StyleAljaafari, H., Gu, Y., Chicchelly, H., & Nuxoll, E. (2021). Thermal Shock and Ciprofloxacin Act Orthogonally on Pseudomonas aeruginosa Biofilms. Antibiotics, 10(8), 1017. https://doi.org/10.3390/antibiotics10081017