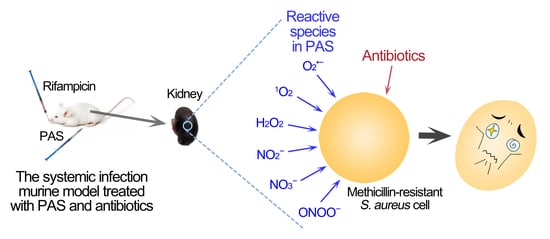
Plasma-Activated Saline Promotes Antibiotic Treatment of Systemic Methicillin-Resistant Staphylococcus aureus Infection
Abstract
:1. Introduction
2. Results
2.1. Reactive Species in PAS
2.2. Biofilm Reduced by the Combination of PAS and Antibiotics In Vitro
2.3. Treatment with PAS and Rifampicin in a Murine Systemic Infection Model
2.4. Biosafety of the Combination Treatment in a Mice Model
3. Discussion
4. Materials and Methods
4.1. Plasma Device and Preparation of Plasma-Activated Saline
4.2. Measurement of Reactive Species
4.3. Biofilm Assay
4.4. Mouse Systemic Infection Model
4.5. Treatment
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Lou, W.Y.; Liu, J.X.; Ding, B.S.; Fan, W.M.; Hong, J. A novel antimicrobial polymer efficiently treats multidrug-resistant MRSA-induced bloodstream infection. Biosci. Rep. 2019, 39, BSR20192354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mera, R.M.; Suaya, J.A.; Amrine-Madsen, H.; Hogea, C.S.; Miller, L.A.; Lu, E.P.; Sahm, D.F.; O’Hara, P.; Acosta, C.J. Increasing Role of Staphylococcus aureus and Community-Acquired Methicillin-Resistant Staphylococcus aureus Infections in the United States: A 10-Year Trend of Replacement and Expansion. Microb. Drug Resist. 2011, 17, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Panlilio, A.L.; Culver, D.H.; Gaynes, R.P.; Banerjee, S.; Henderson, T.S.; Tolson, J.S.; Martone, W.J. Methicillin-resistant Staphylococcus aureus in U.S. hospitals, 1975–1991. Infect. Control Hosp. Epidemiol. 1992, 13, 582–586. [Google Scholar]
- Kokai-Kun, J.F.; Chanturiya, T.; Mond, J.J. Lysostaphin as a treatment for systemic Staphylococcus aureus infection in a mouse model. J. Antimicrob. Chemoth. 2007, 60, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Alam, A.; Rani, M.; Ehtesham, N.Z.; Hasnain, S.E. Biofilms: Survival and defense strategy for pathogens. Int. J. Med. Microbiol. 2017, 307, 481–489. [Google Scholar] [CrossRef]
- Srivastava, S.; Bhargava, A. Biofilms and human health. Biotechnol. Lett. 2016, 38, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzzaman, N.F.; Firdessa, R.; Good, L. Bactericidal effects of polyhexamethylene biguanide against intracellular Staphylococcus aureus EMRSA-15 and USA 300. J. Antimicrob. Chemoth. 2016, 71, 1252–1259. [Google Scholar] [CrossRef] [Green Version]
- Rasigade, J.P.; Moulay, A.; Lhoste, Y.; Tristan, A.; Bes, M.; Vandenesch, F.; Etienne, J.; Lina, G.; Laurent, F.; Dumitrescu, O. Impact of sub-inhibitory antibiotics on fibronectin-mediated host cell adhesion and invasion by Staphylococcus aureus. BMC Microbiol. 2011, 11, 263. [Google Scholar] [CrossRef] [Green Version]
- Loomba, P.S.; Taneja, J.; Mishra, B. Methicillin and Vancomycin Resistant S. aureus in Hospitalized Patients. J. Glob. Infect. Dis. 2010, 2, 275–283. [Google Scholar] [CrossRef]
- Rossi, F.; Diaz, L.; Wollam, A.; Panesso, D.; Zhou, Y.; Rincon, S.; Narechania, A.; Xing, G.; Di Gioia, T.S.R.; Doi, A.; et al. Transferable Vancomycin Resistance in a Community-Associated MRSA Lineage. N. Engl. J. Med. 2014, 370, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Rodvold, K.A.; McConeghy, K.W. Methicillin-Resistant Staphylococcus aureus Therapy: Past, Present, and Future. Clin. Infect. Dis. 2014, 58, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Chhibber, T.; Gondil, V.S.; Sinha, V.R. Development of Chitosan-Based Hydrogel Containing Antibiofilm Agents for the Treatment of Staphylococcus aureus-Infected Burn Wound in Mice. AAPS Pharmscitech 2020, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Wong-Beringer, A.; Joo, J.; Tse, E.; Beringer, P. Vancomycin-associated nephrotoxicity: A critical appraisal of risk with high-dose therapy. Int. J. Antimicrob. Agents 2011, 37, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Waterer, G.; Lord, J.; Hofmann, T.; Jouhikainen, T. Phase I, Dose-Escalating Study of the Safety and Pharmacokinetics of Inhaled Dry-Powder Vancomycin (AeroVanc) in Volunteers and Patients with Cystic Fibrosis: A New Approach to Therapy for Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2020, 64, e01776-19. [Google Scholar] [CrossRef]
- Worthington, R.J.; Melander, C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Dolgin, E. Sequencing of superbugs seen as key to combating their spread. Nat. Med. 2010, 16, 1054. [Google Scholar] [CrossRef]
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination therapy for treatment of infections with gram-negative bacteria. Clin. Microbiol. Rev. 2012, 25, 450–470. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.M.; Saini, V.; Ashbaugh, A.G.; Miller, R.J.; Ordonez, A.A.; Ortines, R.V.; Wang, Y.; Sterling, R.S.; Jain, S.K.; Miller, L.S. Oral-Only Linezolid-Rifampin Is Highly Effective Compared with Other Antibiotics for Periprosthetic Joint Infection Study of a Mouse Model. J. Bone Joint Surg. Am. 2017, 99, 656–665. [Google Scholar] [CrossRef]
- Petrosillo, N.; Ioannidou, E.; Falagas, M.E. Colistin monotherapy vs. combination therapy: Evidence from microbiological, animal and clinical studies. Clin. Microbiol. Infect. 2008, 14, 816–827. [Google Scholar] [CrossRef] [Green Version]
- Crunkhorn, S. Combination therapy combats MRSA. Nat. Rev. Drug Discov. 2016, 15, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, H.; Wang, J.; Yu, J.; Wei, H. A novel chimeric lysin with robust antibacterial activity against planktonic and biofilm methicillin-resistant Staphylococcus aureus. Sci. Rep. 2017, 7, 40182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miro-Canturri, A.; Ayerbe-Algaba, R.; Jimenez-Mejias, M.E.; Pachon, J.; Smani, Y. Efficacy of Lysophosphatidylcholine as Direct Treatment in Combination with Colistin against Acinetobacter baumannii in Murine Severe Infections Models. Antibiotics 2021, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Cheng, H.; Xu, J.W.; Li, F.; Gao, B.A.; Li, Z.; Gao, C.H.; Huo, K.F.; Fu, J.J.; Xiong, W. Silver-loaded nanotubular structures enhanced bactericidal efficiency of antibiotics with synergistic effect in vitro and in vivo. Int. J. Nanomedicine 2017, 12, 731–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.Y.; Lin, Z.H.; Cheng, Y.P.; Chiu, H.Y.; Yeh, N.L.; Wu, T.K.; Wu, J.S. Wound Healing in Streptozotocin-Induced Diabetic Rats Using Atmospheric-Pressure Argon Plasma Jet. Sci. Rep. 2018, 8, 12214. [Google Scholar] [CrossRef]
- Xu, H.; Ma, R.; Zhu, Y.; Du, M.; Zhang, H.; Jiao, Z. A systematic study of the antimicrobial mechanisms of cold atmospheric-pressure plasma for water disinfection. Sci. Total Environ. 2020, 703, 134965. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.Y.; Zhang, Y.Z.; Lv, Y.; Sun, F.S. The key reactive species in the bactericidal process of plasma activated water. J. Phys. D Appl. Phys. 2020, 53, 185207. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Tech. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Li, D.; Liu, D.; Nie, Q.; Li, H.; Chen, H.; Kong, M.G. Array of surface-confined glow discharge at atmospheric helium: Modes and dynamics. Appl. Phys. Lett. 2014, 104, 204101. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Prasad, K.; Fang, Z.; Speight, R.; Bazaka, K.; Ostrikov, K. Cold atmospheric plasma activated water as a prospective disinfectant: The crucial role of peroxynitrite. Green Chem. 2018, 20, 5276–5284. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma-activated water: Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Kong, M.G.; Deng, X.T. Electrically efficient production of a diffuse nonthermal atmospheric plasma. IEEE Trans. Plasma Sci. 2003, 31, 7–18. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, D.; Chen, C.; Li, D.; Yang, A.; Rong, M.; Chen, H.L.; Kong, M.G. Physicochemical processes in the indirect interaction between surface air plasma and deionized water. J. Phys. D Appl. Phys. 2015, 48, 495201. [Google Scholar] [CrossRef]
- Gorbanev, Y.; O’Connell, D.; Chechik, V. Ozone correlates with antibacterial effects from indirect air dielectric barrier discharge treatment of water. Chem. Eur. J. 2016, 22, 3496–3505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Tian, Y.; Li, Y.L.; Ma, R.A.; Zhang, Q.; Zhang, J.; Fang, J. Bactericidal Effects against S-aureus and Physicochemical Properties of Plasma Activated Water stored at different temperatures. Sci. Rep. 2016, 6, 28505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balan, G.G.; Rosca, I.; Ursu, E.L.; Doroftei, F.; Bostanaru, A.C.; Hnatiuc, E.; Nastasa, V.; Sandru, V.; Stefanescu, G.; Trifan, A.; et al. Plasma-activated water: A new and effective alternative for duodenoscope reprocessing. Infect. Drug Resist. 2018, 11, 727–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurita, R.; Contaldo, N.; Zambon, Y.; Bisag, A.; Canel, A.; Gherardi, M.; Laghi, G.; Bertaccini, A.; Colombo, V. The use of plasma-activated water in viticulture: Induction of resistance and agronomic performance in greenhouse and open field. Plasma Process. Polym. 2021, 18, e2000206. [Google Scholar] [CrossRef]
- Wandell, R.J.; Locke, B.R. Hydrogen Peroxide Generation in Low Power Pulsed Water Spray Plasma Reactors. Ind. Eng. Chem. Res. 2014, 53, 609–618. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, R.N.; Tian, Y.; Su, B.; Wang, K.L.; Yu, S.; Zhang, J.; Fang, J. Sterilization Efficiency of a Novel Electrochemical Disinfectant against Staphylococcus aureus. Environ. Sci. Technol. 2016, 50, 3184–3192. [Google Scholar] [CrossRef] [PubMed]
- Vlad, I.E.; Martin, C.; Toth, A.R.; Papp, J.; Anghel, S.D. Bacterial Inhibition Effect of Plasma Activated Water. Rom. Rep. Phys. 2019, 71, 602. [Google Scholar]
- Charoux, C.M.G.; Patange, A.D.; Hinds, L.M.; Simpson, J.C.; O’Donnell, C.P.; Tiwari, B.K. Antimicrobial effects of airborne acoustic ultrasound and plasma activated water from cold and thermal plasma systems on biofilms. Sci. Rep. 2020, 10, 17297. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Tian, Y.; Zhou, H.Z.; Li, Y.L.; Zhang, Z.H.; Jiang, B.Y.; Yang, B.; Zhang, J.; Fang, J. Inactivation Efficacy of Nonthermal Plasma-Activated Solutions against Newcastle Disease Virus. Appl. Environ. Microbiol. 2018, 84, e02836-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.Y.; Qu, K.; Zhang, X.; Wang, B.C.; Wang, W.T.; Bi, J.B.; Zhang, S.M.; Li, Z.Y.; Kong, M.G.; Liu, D.X.; et al. Discharge Plasma-Activated Saline Protects against Abdominal Sepsis by Promoting Bacterial Clearance. Shock 2019, 52, 92–101. [Google Scholar] [CrossRef]
- Takeda, S.; Yamada, S.; Hattori, N.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Kobayashi, D.; Tanaka, C.; Fujii, T.; et al. Intraperitoneal Administration of Plasma-Activated Medium: Proposal of a Novel Treatment Option for Peritoneal Metastasis From Gastric Cancer. Ann. Surg. Oncol. 2017, 24, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xu, R.B.; Zhao, Y.M.; Liu, D.X.; Liu, Z.J.; Wang, X.H.; Chen, H.L.; Kong, M.G. Gas Plasma Pre-treatment Increases Antibiotic Sensitivity and Persister Eradication in Methicillin-Resistant Staphylococcus aureus. Front. Microbiol. 2018, 9, 537. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chin, W.; Dong, H.; Xu, L.; Zhong, G.; Huang, Y.; Li, L.; Xu, K.; Wu, M.; Hedrick, J.L.; et al. Biodegradable Antimicrobial Polycarbonates with In Vivo Efficacy against Multidrug-Resistant MRSA Systemic Infection. Adv. Healthc. Mater. 2015, 4, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Methicillin-resistant Staphylococcus aureus infection is associated with increased mortality. Future Microbiol. 2012, 7, 189–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, P.L.; Lo, P.Y.; Chow, K.H.; Lau, E.H.; Lai, E.L.; Cheng, V.C.; Kao, R.Y. Vancomycin MIC creep in MRSA isolates from 1997 to 2008 in a healthcare region in Hong Kong. J. Infect. 2010, 60, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Tsiodras, S.; Gold, H.S.; Sakoulas, G.; Eliopoulos, G.M.; Wennersten, C.; Venkataraman, L.; Moellering, R.C.; Ferraro, M.J. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 2001, 358, 207–208. [Google Scholar] [CrossRef]
- Van Hal, S.J.; Lodise, T.P.; Paterson, D.L. The Clinical Significance of Vancomycin Minimum Inhibitory Concentration in Staphylococcus aureus Infections: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2012, 54, 755–771. [Google Scholar] [CrossRef] [Green Version]
- Melo-Cristino, J.; Resina, C.; Manuel, V.; Lito, L.; Ramirez, M. First case of infection with vancomycin-resistant Staphylococcus aureus in Europe. Lancet 2013, 382, 205. [Google Scholar] [CrossRef]
- Hua, X.; Yang, Q.; Zhang, W.; Dong, Z.; Yu, S.; Schwarz, S.; Liu, S. Antibacterial Activity and Mechanism of Action of Aspidinol Against Multi-Drug-Resistant Methicillin-Resistant Staphylococcus aureus. Front. Pharmacol. 2018, 9, 619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Sobieraj, A.M.; Huemer, M.; Zinsli, L.V.; Meile, S.; Keller, A.P.; Rohrig, C.; Eichenseher, F.; Shen, Y.; Zinkernagel, A.S.; Loessner, M.J.; et al. Engineering of Long-Circulating Peptidoglycan Hydrolases Enables Efficient Treatment of Systemic Staphylococcus aureus Infection. Mbio 2020, 11, e01781-20. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O. Contributions of reactive oxygen species, oxidative DNA damage and glutathione depletion to the sensitivity of Acinetobacter baumannii to 2-(2-nitrovinyl) furan. Microb. Pathog. 2019, 128, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, M.; Kuroiwa, Y.; Inoue, T.; Hibi, D.; Matsushita, K.; Ishii, Y.; Maruyama, S.; Nohmi, T.; Nishikawa, A.; Umemura, T. Oxidative DNA damage and in vivo mutagenicity caused by reactive oxygen species generated in the livers of p53-proficient or -deficient gpt delta mice treated with non-genotoxic hepatocarcinogens. J. Appl.Toxicol. 2013, 33, 1433–1441. [Google Scholar] [CrossRef]
- He, L.-L.; Wang, X.; Wu, X.-X.; Wang, Y.-X.; Kong, Y.-M.; Wang, X.; Liu, B.-M.; Liu, B. Protein damage and reactive oxygen species generation induced by the synergistic effects of ultrasound and methylene blue. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Downs, D.M.; Ernst, D.C. From microbiology to cancer biology: The Rid protein family prevents cellular damage caused by endogenously generated reactive nitrogen species. Mol. Microbiol. 2015, 96, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.H.; Wang, S.; Li, B.; Qi, M.; Feng, R.; Li, Q.S.; Zhang, H.; Chen, H.L.; Kong, M.G. Effects of Plasma-Activated Water on Skin Wound Healing in Mice. Microorganisms 2020, 8, 1091. [Google Scholar] [CrossRef]
- Zou, X.Y.; Xu, M.Y.; Pan, S.H.; Gan, L.; Zhang, S.; Chen, H.X.; Liu, D.W.; Lu, X.P.; Ostrikov, K.K. Plasma Activated Oil: Fast Production, Reactivity, Stability, and Wound Healing Application. ACS Biomater. Sci. Eng. 2019, 5, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, M.; Merbahi, N.; Pathak, A.; Eichwald, O. Low-temperature plasmas at atmospheric pressure: Toward new pharmaceutical treatments in medicine. Fundam. Clin. Pharmacol. 2014, 28, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Azzariti, A.; Iacobazzi, R.M.; Di Fonte, R.; Porcelli, L.; Gristina, R.; Favia, P.; Fracassi, F.; Trizio, I.; Silvestris, N.; Guida, G.; et al. Plasma-activated medium triggers cell death and the presentation of immune activating danger signals in melanoma and pancreatic cancer cells. Sci. Rep. 2019, 9, 4099. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, J.; Han, G.; Liu, Z.; Liu, C.; Zhang, C.; Liu, B.; Jiang, C.; Liu, R.; Zhao, T.; et al. Real-Time Discrimination and Versatile Profiling of Spontaneous Reactive Oxygen Species in Living Organisms with a Single Fluorescent Probe. J. Am. Chem. Soc. 2016, 138, 3769–3778. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xianyu, Y.; Wang, N.; Yan, Z.; Liu, Y.; Zhu, K.; Hatzakis, N.S.; Jiang, X. Functionalized Gold Nanoclusters Identify Highly Reactive Oxygen Species in Living Organisms. Adv. Funct. Mater. 2018, 28, 1702026. [Google Scholar] [CrossRef]
- Guo, L.; Xu, R.B.; Liu, D.X.; Qi, Y.; Guo, Y.H.; Wang, W.T.; Zhang, J.; Liu, Z.J.; Kong, M.G. Eradication of methicillin-resistant Staphylococcus aureus biofilms by surface discharge plasmas with various working gases. J. Phys. D Appl. Phys. 2019, 52, 425202. [Google Scholar] [CrossRef]





| Parameter | Non-Infected | Non-Infected and Treated with PAS | Non-Infected and Treated with PAS and Rifampicin | Infected | Infected and Treated with PAS | Infected and Treated with PAS and Rifampicin |
|---|---|---|---|---|---|---|
| RBC (×1012/L) | 11.23 ± 0.56 | 11.33 ± 0.88 | 11.44 ± 0.31 | 11.23 ± 0.94 | 11.68 ± 0.64 | 11.14 ± 0.72 |
| WBC (×109/L) | 5.32 ± 0.87 | 7.28 ± 2.70 | 6.48 ± 1.25 | 7.94 ± 0.87 a | 5.42 ± 1.37 b | 5.56 ± 1.13 b |
| Neutrophils (%) | 36.62 ± 6.47 | 32.32 ± 6.62 | 36.38 ± 4.86 | 50.70 ± 8.02 a | 38.56 ± 3.46 b | 31.56 ± 6.53 b |
| Lymphocytes (%) | 59.62 ± 7.12 | 63.72 ± 6.74 | 60.76 ± 4.83 | 42.74 ± 8.53 a | 57.00 ± 3.64 b | 65.06 ± 6.35 b,c |
| Monocytes (%) | 3.76 ± 0.88 | 3.96 ± 0.89 | 3.88 ± 0.96 | 6.56 ± 1.16 a | 4.44 ± 0.29 b | 4.58 ± 0.68 b |
| MCV (fL) | 49.80 ± 0.95 | 50.22 ± 1.04 | 49.42 ± 0.51 | 49.08 ± 0.70 | 48.84 ± 0.94 | 48.46 ± 1.35 |
| MCH (pg) | 14.90 ± 0.39 | 14.84 ± 0.28 | 14.76 ± 0.19 | 14.62 ± 0.45 | 14.68 ± 0.36 | 14.64 ± 0.32 |
| MCHC (g/L) | 299.80 ± 3.11 | 296.60 ± 7.13 | 299.00 ± 0.71 | 296.00 ± 2.83 | 302.00 ± 7.52 | 304.40 ± 7.54 |
| PLT (×109/L) | 1330.0 ± 177.5 | 1455.2 ± 201.4 | 1584.6 ± 87.9 | 2480.0 ± 277.8 a | 2613.0 ± 258.6 a | 2649.4 ± 620.3 a |
| HGB (g/L) | 168.0 ± 11.9 | 168.8 ± 14.7 | 169.2 ± 4.3 | 162.6 ± 14.0 | 172.4 ± 11.2 | 163.8 ± 11.3 |
| Hematocrit (%) | 55.92 ± 3.74 | 56.80 ± 4.15 | 56.46 ± 1.52 | 54.62 ± 4.43 | 57.02 ± 3.79 | 54.00 ± 4.51 |
| Parameter | Non-Infected | Non-Infected and Treated with PAS | Non-Infected and Treated with PAS and Rifampicin | Infected | Infected and Treated with PAS | Infected and Treated with PAS and Rifampicin |
|---|---|---|---|---|---|---|
| Liver | ||||||
| AST (U/L) | 144.24 ± 10.78 | 163.72 ± 23.42 | 151.73 ± 11.97 | 169.40 ± 31.74 | 161.17 ± 15.46 | 163.09 ± 12.91 |
| ALT (U/L) | 49.76 ± 13.00 | 48.17 ± 6.43 | 56.36 ± 14.01 | 50.37 ± 8.31 | 58.68 ± 8.27 | 58.82 ± 3.75 |
| ALP (U/L) | 189.18 ± 27.75 | 161.47 ± 22.69 | 176.53 ± 34.60 | 93.60 ± 29.38 a | 123.20 ± 13.36 a | 125.35 ± 27.03 a |
| T-BIL (μmol/L) | 21.32 ± 5.99 | 22.34 ± 3.89 | 21.70 ± 1.99 | 15.96 ± 3.13 | 16.96 ± 3.44 | 21.96 ± 7.25 |
| ALB (g/L) | 39.24 ± 2.20 | 37.26 ± 2.03 | 37.51 ± 2.55 | 32.53 ± 1.94 a | 34.40 ± 1.21 a | 36.66 ± 1.39 b |
| Kidney | ||||||
| BUN (mmol/L) | 25.79 ± 2.90 | 24.71 ± 3.30 | 25.54 ± 3.96 | 30.41 ± 7.95 | 24.72 ± 4.11 | 31.91 ± 6.90 |
| UA (μmol/L) | 153.72 ± 38.87 | 228.70 ± 94.52 | 202.71 ± 50.36 | 375.59 ± 48.69 a | 221.07 ± 37.45 a,b | 226.78 ± 97.29 b |
| CREA (μmol/L) | 24.11 ± 7.70 | 27.33 ± 5.78 | 21.45 ± 2.53 | 31.08 ± 4.11 | 26.79 ± 1.91 | 31.96 ± 8.92 |
| Heart | ||||||
| LDH (U/L) | 2300.82 ± 356.61 | 2835.74 ± 734.15 | 2795.73 ± 755.79 | 3125.76 ± 837.71 | 2698.76 ± 429.21 | 2918.54 ± 866.03 |
| CK (U/L) | 1020.00 ± 276.44 | 1428.94 ± 458.92 | 1295.14 ± 170.85 | 1517.87 ± 514.50 | 1231.48 ± 224.05 | 1274.19 ± 154.97 |
| Glucose | ||||||
| GLU (mmol/L) | 1.05 ± 0.50 | 0.92 ± 0.34 | 1.21 ± 0.14 | 0.92 ± 0.63 | 0.94 ± 0.84 | 0.97 ± 0.60 |
| GSP (mmol/L) | 4.51 ± 0.37 | 4.50 ± 0.30 | 4.30 ± 0.50 | 2.39 ± 0.31 a | 3.32 ± 0.24 a,b | 3.82 ± 0.30 a,b,c |
| Lipid | ||||||
| TG (mmol/L) | 1.36 ± 0.51 | 1.19 ± 0.28 | 1.25 ± 0.26 | 0.60 ± 0.13 a | 0.66 ± 0.08 a | 0.66 ± 0.22 a |
| T-CHO (mmol/L) | 1.74 ± 0.47 | 2.01 ± 0.19 | 1.90 ± 0.30 | 2.41 ± 0.49 | 1.97 ± 0.29 | 2.23 ± 0.28 |
| Other | ||||||
| TP (g/L) | 50.06 ± 2.22 | 53.30 ± 4.48 | 50.64 ± 1.78 | 51.32 ± 0.85 | 50.32 ± 1.67 | 52.15 ± 5.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Niyazi, G.; Qi, Y.; Yao, Z.; Huang, L.; Wang, Z.; Guo, L.; Liu, D. Plasma-Activated Saline Promotes Antibiotic Treatment of Systemic Methicillin-Resistant Staphylococcus aureus Infection. Antibiotics 2021, 10, 1018. https://doi.org/10.3390/antibiotics10081018
Yang L, Niyazi G, Qi Y, Yao Z, Huang L, Wang Z, Guo L, Liu D. Plasma-Activated Saline Promotes Antibiotic Treatment of Systemic Methicillin-Resistant Staphylococcus aureus Infection. Antibiotics. 2021; 10(8):1018. https://doi.org/10.3390/antibiotics10081018
Chicago/Turabian StyleYang, Lu, Gulimire Niyazi, Yu Qi, Zhiqian Yao, Lingling Huang, Zifeng Wang, Li Guo, and Dingxin Liu. 2021. "Plasma-Activated Saline Promotes Antibiotic Treatment of Systemic Methicillin-Resistant Staphylococcus aureus Infection" Antibiotics 10, no. 8: 1018. https://doi.org/10.3390/antibiotics10081018
APA StyleYang, L., Niyazi, G., Qi, Y., Yao, Z., Huang, L., Wang, Z., Guo, L., & Liu, D. (2021). Plasma-Activated Saline Promotes Antibiotic Treatment of Systemic Methicillin-Resistant Staphylococcus aureus Infection. Antibiotics, 10(8), 1018. https://doi.org/10.3390/antibiotics10081018