In Silico and In Vitro Analysis of Sulforaphane Anti-Candida Activity
Abstract
:1. Introduction
2. Results
2.1. In Silico Analysis
2.1.1. Analysis of Biological Activity
2.1.2. Estimated Oral Bioavailability and Expected Toxicity
2.2. In Vitro Analysis
2.2.1. In Vitro Antifungal Activity
2.2.2. In Vitro Evaluation of the Combined Effects of SFN and FLZ
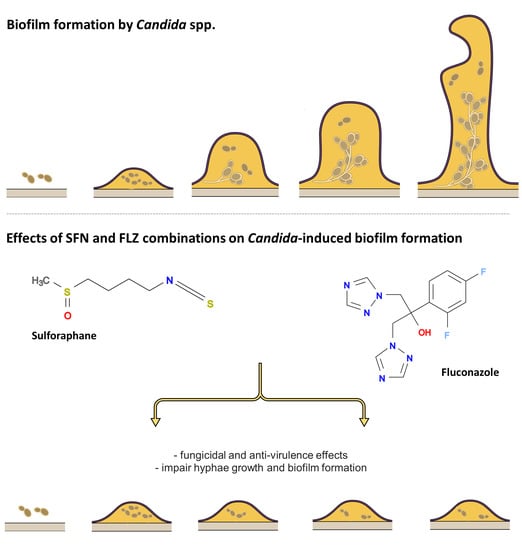
2.2.3. Effects on Hyphae Formation
2.2.4. Effects on Biofilm Formation
2.2.5. Effects on mRNA Expression of Hyphae Growth- and Biofilm Formation-Related Genes
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis
4.1.1. Prediction of Biological Activities
4.1.2. Prediction of Oral Bioavailability
4.1.3. Estimations of Toxicity and Pharmacokinetic Characteristics
4.2. In Vitro Analysis
4.2.1. Microorganisms
4.2.2. Inoculum Preparation
4.2.3. Determination of Minimum Inhibitory (MIC) and Fungicidal (MFC) Concentrations
4.2.4. Combined Effects of SFN with FLZ on Fungal Survival
4.2.5. Biofilm Formation Assay
4.2.6. Hyphae Formation Test
4.2.7. mRNA Expression of Hyphae Growth- and Biofilm Formation-Related Genes
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spalanzani, R.N.; Mattos, K.; Marques, L.I.; Barros, P.F.D.; Pereira, P.I.P.; Paniago, A.M.M.; Mendes, R.P.; Chang, M.R. Clinical and laboratorial features of oral candidiasis in HIV-positive patients. Rev. Soc. Bras. Med. Trop. 2018, 51, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Fidel, P.L., Jr. Candida-host interactions in HIV disease: Implications for oropharyngeal candidiasis. Adv. Dent. Res. 2011, 23, 45–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, P.P.; Saikia, L.; Nath, R.; Phukan, S.K. Species distribution & antifungal susceptibility pattern of oropharyngeal Candida isolates from human immunodeficiency virus infected individuals. Indian J. Med. Res. 2016, 143, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Khedri, S.; Santos, A.L.S.; Roudbary, M.; Hadighi, R.; Falahati, M.; Farahyar, S.; Khoshmirsafa, M.; Kalantari, S. Iranian HIV/AIDS patients with oropharyngeal candidiasis: Identification, prevalence and antifungal susceptibility of Candida species. Lett. Appl. Microbiol. 2018, 67, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Hosain Pour, A.; Salari, S.; Ghasemi Nejad Almani, P. Oropharyngeal candidiasis in HIV/AIDS patients and non-HIV subjects in the Southeast of Iran. Curr. Med. Mycol. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Salari, S.; Khosravi, A.R.; Mousavi, S.A.; Nikbakht-Brojeni, G.H. Mechanisms of resistance to fluconazole in Candida albicans clinical isolates from Iranian HIV-infected patients with oropharyngeal candidiasis. J. Mycol. Med. 2016, 26, 35–41. [Google Scholar] [CrossRef]
- Hellstein, J.W.; Marek, C.L. Candidiasis: Red and White Manifestations in the Oral Cavity. Head Neck Pathol. 2019, 13, 25–32. [Google Scholar] [CrossRef]
- Patil, S.; Majumdar, B.; Sarode, S.C.; Sarode, G.S.; Awan, K.H. Oropharyngeal Candidosis in HIV-Infected Patients-An Update. Front. Microbiol. 2018, 9, 980. [Google Scholar] [CrossRef] [Green Version]
- Goulart, L.S.; Souza, W.W.R.; Vieira, C.A.; Lima, J.S.; Olinda, R.A.; Araujo, C. Oral colonization by Candida species in HIV-positive patients: Association and antifungal susceptibility study. Einstein 2018, 16, eAO4224. [Google Scholar] [CrossRef]
- Hu, L.; He, C.; Zhao, C.; Chen, X.; Hua, H.; Yan, Z. Characterization of oral candidiasis and the Candida species profile in patients with oral mucosal diseases. Microb. Pathog. 2019, 134, 103575. [Google Scholar] [CrossRef]
- Lortholary, O.; Petrikkos, G.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Calandra, T.; Castagnola, E.; Cornely, O.A.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Patients with HIV infection or AIDS. Clin. Microbiol. Infect. 2012, 18, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Ellis, D.; Tullio, V.; Rodloff, A.; Fu, W.; Ling, T.A.; Global Antifungal Surveillance Group. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: A 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 2010, 48, 1366–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajadurai, S.G.; Maharajan, M.K.; Veettil, S.K.; Gopinath, D. Comparative Efficacy of Antifungal Agents Used in the Treatment of Oropharyngeal Candidiasis among HIV-Infected Adults: A Systematic Review and Network Meta-Analysis. J. Fungi 2021, 7, 637. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.E.; Bicanic, T. Drug Resistance and Novel Therapeutic Approaches in Invasive Candidiasis. Front. Cell Infect. Microbiol. 2021, 11, 759408. [Google Scholar] [CrossRef]
- Badiee, P.; Badali, H.; Boekhout, T.; Diba, K.; Moghadam, A.G.; Hossaini Nasab, A.; Jafarian, H.; Mohammadi, R.; Mirhendi, H.; Najafzadeh, M.J.; et al. Antifungal susceptibility testing of Candida species isolated from the immunocompromised patients admitted to ten university hospitals in Iran: Comparison of colonizing and infecting isolates. BMC Infect. Dis. 2017, 17, 727. [Google Scholar] [CrossRef] [Green Version]
- Dehghani Nazhvani, A.; Haddadi, P.; Badiee, P.; Malekhoseini, S.A.; Jafarian, H. Antifungal Effects of Common Mouthwashes on Candida Strains Colonized in the Oral Cavities of Liver Transplant Recipients in South Iran in 2014. Hepat. Mon. 2016, 16, e31245. [Google Scholar] [CrossRef] [Green Version]
- Shivaswamy, U.; Sumana, M.N. Antifungal Resistance of Candida Species Isolated from HIV Patients in a Tertiary Care Hospital, Mysuru, Karnataka. Indian J. Dermatol. 2020, 65, 423–425. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Rhomberg, P.R.; Messer, S.A.; Jones, R.N.; Castanheira, M. Isavuconazole, micafungin, and 8 comparator antifungal agents’ susceptibility profiles for common and uncommon opportunistic fungi collected in 2013: Temporal analysis of antifungal drug resistance using CLSI species-specific clinical breakpoints and proposed epidemiological cutoff values. Diagn. Microbiol. Infect. Dis. 2015, 82, 303–313. [Google Scholar] [CrossRef]
- Sandai, D.; Tabana, Y.M.; Ouweini, A.E.; Ayodeji, I.O. Resistance of Candida albicans Biofilms to Drugs and the Host Immune System. Jundishapur J. Microbiol. 2016, 9, e37385. [Google Scholar] [CrossRef] [Green Version]
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013, 8, 1325–1337. [Google Scholar] [CrossRef]
- De Figueiredo, S.M.; Filho, S.A.; Nogueira-Machado, J.A.; Caligiorne, R.B. The anti-oxidant properties of isothiocyanates: A review. Recent Pat. Endocr. Metab. Immune Drug Discov. 2013, 7, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurepina, N.; Kreiswirth, B.N.; Mustaev, A. Growth-inhibitory activity of natural and synthetic isothiocyanates against representative human microbial pathogens. J. Appl. Microbiol. 2013, 115, 943–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowicki, D.; Rodzik, O.; Herman-Antosiewicz, A.; Szalewska-Palasz, A. Isothiocyanates as effective agents against enterohemorrhagic Escherichia coli: Insight to the mode of action. Sci. Rep. 2016, 6, 22263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaszko, T.; Szucs, Z.; Vasas, G.; Gonda, S. Effects of Glucosinolate-Derived Isothiocyanates on Fungi: A Comprehensive Review on Direct Effects, Mechanisms, Structure-Activity Relationship Data and Possible Agricultural Applications. J. Fungi 2021, 7, 539. [Google Scholar] [CrossRef]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Kim, B.; Kim, S.H.; Srivastava, S.K. Molecular targets of isothiocyanates in cancer: Recent advances. Mol. Nutr. Food Res. 2014, 58, 1685–1707. [Google Scholar] [CrossRef] [Green Version]
- Prawan, A.; Saw, C.L.; Khor, T.O.; Keum, Y.S.; Yu, S.; Hu, L.; Kong, A.N. Anti-NF-kappaB and anti-inflammatory activities of synthetic isothiocyanates: Effect of chemical structures and cellular signaling. Chem. Biol. Interact. 2009, 179, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A.E.; Terschluesen, A.M.; Rimbach, G. Health promoting effects of brassica-derived phytochemicals: From chemopreventive and anti-inflammatory activities to epigenetic regulation. Oxid. Med. Cell. Longev. 2013, 2013, 964539. [Google Scholar] [CrossRef] [Green Version]
- Cheung, K.L.; Kong, A.N. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010, 12, 87–97. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxid. Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, T.; Xu, F.; Yan, X.; Li, S.; Li, H. Sulforaphane exerts anti-inflammatory effects against lipopolysaccharide-induced acute lung injury in mice through the Nrf2/ARE pathway. Int. J. Mol. Med. 2016, 37, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, M.R.; de Bittencourt Brasil, F.; Furstenau, C.R. Sulforaphane Promotes Mitochondrial Protection in SH-SY5Y Cells Exposed to Hydrogen Peroxide by an Nrf2-Dependent Mechanism. Mol. Neurobiol. 2018, 55, 4777–4787. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Atkinson, S.J.; Akbareian, S.E.; Zhou, Z.; Munsterberg, A.; Robinson, S.D.; Bao, Y. Sulforaphane exerts anti-angiogenesis effects against hepatocellular carcinoma through inhibition of STAT3/HIF-1alpha/VEGF signalling. Sci. Rep. 2017, 7, 12651. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Spagnuolo, C.; Russo, G.L.; Skalicka-Wozniak, K.; Daglia, M.; Sobarzo-Sanchez, E.; Nabavi, S.F.; Nabavi, S.M. Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Crit. Rev. Food Sci. Nutr. 2018, 58, 1391–1405. [Google Scholar] [CrossRef]
- Su, X.; Jiang, X.; Meng, L.; Dong, X.; Shen, Y.; Xin, Y. Anticancer Activity of Sulforaphane: The Epigenetic Mechanisms and the Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 5438179. [Google Scholar] [CrossRef] [Green Version]
- Angeloni, C.; Malaguti, M.; Rizzo, B.; Barbalace, M.C.; Fabbri, D.; Hrelia, S. Neuroprotective effect of sulforaphane against methylglyoxal cytotoxicity. Chem. Res. Toxicol. 2015, 28, 1234–1245. [Google Scholar] [CrossRef]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013, 2013, 415078. [Google Scholar] [CrossRef] [Green Version]
- Houghton, C.A. Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxid. Med. Cell. Longev. 2019, 2019, 2716870. [Google Scholar] [CrossRef] [Green Version]
- Haristoy, X.; Angioi-Duprez, K.; Duprez, A.; Lozniewski, A. Efficacy of sulforaphane in eradicating Helicobacter pylori in human gastric xenografts implanted in nude mice. Antimicrob. Agents Chemother. 2003, 47, 3982–3984. [Google Scholar] [CrossRef]
- Yanaka, A.; Fahey, J.W.; Fukumoto, A.; Nakayama, M.; Inoue, S.; Zhang, S.; Tauchi, M.; Suzuki, H.; Hyodo, I.; Yamamoto, M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev. Res. 2009, 2, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aires, A.; Mota, V.R.; Saavedra, M.J.; Rosa, E.A.; Bennett, R.N. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J. Appl. Microbiol. 2009, 106, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Johansson, N.L.; Pavia, C.S.; Chiao, J.W. Growth inhibition of a spectrum of bacterial and fungal pathogens by sulforaphane, an isothiocyanate product found in broccoli and other cruciferous vegetables. Planta Med. 2008, 74, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Cierpial, T.; Kielbasinski, P.; Kwiatkowska, M.; Lyzwa, P.; Lubelska, K.; Kuran, D.; Dabrowska, A.; Kruszewska, H.; Mielczarek, L.; Chilmonczyk, Z.; et al. Fluoroaryl analogs of sulforaphane—A group of compounds of anticancer and antimicrobial activity. Bioorg. Chem. 2020, 94, 103454. [Google Scholar] [CrossRef]
- Devi, J.R.; Thangam, E.B. Studies on antioxidant and antimicrobial activities of purified sulforaphane from Brassica oleraceae var. rubra. J. Pharm. Res. 2012, 5, 3582–3584. [Google Scholar]
- Murata, W.; Yamaguchi, Y.; Fujita, K.I.; Yamauchi, K.; Tanaka, T.; Ogita, A. Enhancement of paraben-fungicidal activity by sulforaphane, a cruciferous vegetable-derived isothiocyanate, via membrane structural damage in Saccharomyces cerevisiae. Lett. Appl. Microbiol. 2019, 69, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.V. Candida albicans Hyphae: From Growth Initiation to Invasion. J. Fungi 2018, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Yong, J.; Zu, R.; Huang, X.; Ge, Y.; Li, Y. Synergistic Effect of Berberine Hydrochloride and Fluconazole against Candida albicans Resistant Isolates. Front. Microbiol. 2020, 11, 1498. [Google Scholar] [CrossRef]
- Nett, J.E.; Andes, D.R. Antifungal agents: Spectrum of activity, pharmacology, and clinical indications. Infect. Dis. Clin. 2016, 30, 51–83. [Google Scholar] [CrossRef]
- Abel, E.L.; Boulware, S.; Fields, T.; McIvor, E.; Powell, K.L.; DiGiovanni, J.; Vasquez, K.M.; MacLeod, M.C. Sulforaphane induces phase II detoxication enzymes in mouse skin and prevents mutagenesis induced by a mustard gas analog. Toxicol. Appl. Pharmacol. 2013, 266, 439–442. [Google Scholar] [CrossRef] [Green Version]
- Huo, L.; Su, Y.; Xu, G.; Zhai, L.; Zhao, J. Sulforaphane Protects the Male Reproductive System of Mice from Obesity-Induced Damage: Involvement of Oxidative Stress and Autophagy. Int. J. Environ. Res. Public Health 2019, 16, 3759. [Google Scholar] [CrossRef]
- Sestili, P.; Paolillo, M.; Lenzi, M.; Colombo, E.; Vallorani, L.; Casadei, L.; Martinelli, C.; Fimognari, C. Sulforaphane induces DNA single strand breaks in cultured human cells. Mutat. Res. 2010, 689, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chang, L.; Liu, G.; Wang, X.; Yang, Y.; Hashimoto, K. Long-lasting beneficial effects of maternal intake of sulforaphane glucosinolate on gut microbiota in adult offspring. J. Nutr. Biochem. 2022, 109, 109098. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.D.; Hsu, A.; Williams, D.E.; Dashwood, R.H.; Stevens, J.F.; Yamamoto, M.; Ho, E. Metabolism and tissue distribution of sulforaphane in Nrf2 knockout and wild-type mice. Pharm. Res. 2011, 28, 3171–3179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jazwa, A.; Rojo, A.I.; Innamorato, N.G.; Hesse, M.; Fernandez-Ruiz, J.; Cuadrado, A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid. Redox Signal. 2011, 14, 2347–2360. [Google Scholar] [CrossRef] [Green Version]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Debruyne, D. Clinical pharmacokinetics of fluconazole in superficial and systemic mycoses. Clin. Pharmacokinet. 1997, 33, 52–77. [Google Scholar] [CrossRef]
- Pappas, P.G.; Chetchotisakd, P.; Larsen, R.A.; Manosuthi, W.; Morris, M.I.; Anekthananon, T.; Sungkanuparph, S.; Supparatpinyo, K.; Nolen, T.L.; Zimmer, L.O.; et al. A phase II randomized trial of amphotericin B alone or combined with fluconazole in the treatment of HIV-associated cryptococcal meningitis. Clin. Infect. Dis. 2009, 48, 1775–1783. [Google Scholar] [CrossRef] [Green Version]
- Thaler, F.; Bernard, B.; Tod, M.; Jedynak, C.P.; Petitjean, O.; Derome, P.; Loirat, P. Fluconazole penetration in cerebral parenchyma in humans at steady state. Antimicrob. Agents Chemother. 1995, 39, 1154–1156. [Google Scholar] [CrossRef] [Green Version]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef] [Green Version]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Bansode, B.S.; Jadhav, A.K.; Karuppayil, S.M. Activity of Allyl Isothiocyanate and Its Synergy with Fluconazole against Candida albicans Biofilms. J. Microbiol. Biotechnol. 2017, 27, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Ganin, H.; Rayo, J.; Amara, N.; Levy, N.; Krief, P.; Meijler, M.M. Sulforaphane and erucin, natural isothiocyanates from broccoli, inhibit bacterial quorum sensing. MedChemComm 2013, 4, 175–179. [Google Scholar] [CrossRef]
- Grainha, T.R.R.; Jorge, P.; Perez-Perez, M.; Perez Rodriguez, G.; Pereira, M.; Lourenco, A.M.G. Exploring anti-quorum sensing and anti-virulence based strategies to fight Candida albicans infections: An in silico approach. FEMS Yeast Res. 2018, 18, foy022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Q.; Summers, E.; Guo, B.; Fink, G. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 1999, 181, 6339–6346. [Google Scholar] [CrossRef] [Green Version]
- Roudbarmohammadi, S.; Roudbary, M.; Bakhshi, B.; Katiraee, F.; Mohammadi, R.; Falahati, M. ALS1 and ALS3 gene expression and biofilm formation in Candida albicans isolated from vulvovaginal candidiasis. Adv. Biomed. Res. 2016, 5, 105. [Google Scholar] [CrossRef]
- Sohn, K.; Urban, C.; Brunner, H.; Rupp, S. EFG1 is a major regulator of cell wall dynamics in Candida albicans as revealed by DNA microarrays. Mol. Microbiol. 2003, 47, 89–102. [Google Scholar] [CrossRef]
- Liu, X.; Li, T.; Wang, D.; Yang, Y.; Sun, W.; Liu, J.; Sun, S. Synergistic Antifungal Effect of Fluconazole Combined with Licofelone against Resistant Candida albicans. Front. Microbiol. 2017, 8, 2101. [Google Scholar] [CrossRef]
- Wu, J.; Wu, D.; Zhao, Y.; Si, Y.; Mei, L.; Shao, J.; Wang, T.; Yan, G.; Wang, C. Sodium New Houttuyfonate Inhibits Candida albicans Biofilm Formation by Inhibiting the Ras1-cAMP-Efg1 Pathway Revealed by RNA-seq. Front. Microbiol. 2020, 11, 2075. [Google Scholar] [CrossRef]
- Ferreira, S.B.; Dantas, T.B.; de Figueredo Silva, D.; Ferreira, P.B.; de Melo, T.R.; de Oliveira Lima, E. In Silico and In Vitro Investigation of the Antifungal Activity of Isoeugenol against Penicillium citrinum. Curr. Top. Med. Chem. 2018, 18, 2186–2196. [Google Scholar] [CrossRef]
- Khurana, N.; Ishar, M.P.; Gajbhiye, A.; Goel, R.K. PASS assisted prediction and pharmacological evaluation of novel nicotinic analogs for nootropic activity in mice. Eur. J. Pharmacol. 2011, 662, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Neto, V.; de Souza, C.D.; Gonzaga, L.F.; da Silveira, B.C.; Sousa, N.C.F.; Pontes, J.P.; Santos, D.M.; Martins, W.C.; Pessoa, J.F.V.; Carvalho Junior, A.R.; et al. Cuminaldehyde potentiates the antimicrobial actions of ciprofloxacin against Staphylococcus aureus and Escherichia coli. PLoS ONE 2020, 15, e0232987. [Google Scholar] [CrossRef] [PubMed]
- Drwal, M.N.; Banerjee, P.; Dunkel, M.; Wettig, M.R.; Preissner, R. ProTox: A web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res. 2014, 42, W53–W58. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard. M27-A3, 3rd ed.; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Santos, J.R.; Gouveia, L.F.; Taylor, E.L.; Resende-Stoianoff, M.A.; Pianetti, G.A.; Cesar, I.C.; Santos, D.A. Dynamic interaction between fluconazole and amphotericin B against Cryptococcus gattii. Antimicrob. Agents Chemother. 2012, 56, 2553–2558. [Google Scholar] [CrossRef] [Green Version]
- Gómez-López, A.; Cuenca-Estrella, M.; Mellado, E.; Rodríguez-Tudela, J.L. In vitro evaluation of combination of terbinafine with itraconazole or amphotericin B against Zygomycota. Diagn. Microbiol. Infect. Dis. 2003, 45, 199–202. [Google Scholar] [CrossRef]
- Atiencia-Carrera, M.B.; Cabezas-Mera, F.S.; Vizuete, K.; Debut, A.; Tejera, E.; Machado, A. Evaluation of the biofilm life cycle between Candida albicans and Candida tropicalis. Front. Cell. Infect. Microbiol. 2022, 12, 953168. [Google Scholar] [CrossRef]
- Ha, K.C.; White, T.C. Effects of azole antifungal drugs on the transition from yeast cells to hyphae in susceptible and resistant isolates of the pathogenic yeast Candida albicans. Antimicrob. Agents Chemother. 1999, 43, 763–768. [Google Scholar] [CrossRef] [Green Version]
- Tsang, P.W.; Bandara, H.M.; Fong, W.P. Purpurin suppresses Candida albicans biofilm formation and hyphal development. PLoS ONE 2012, 7, e50866. [Google Scholar] [CrossRef] [Green Version]
- Roth, R.; Madhani, H.D.; Garcia, J.F. Total RNA Isolation and Quantification of Specific RNAs in Fission Yeast. Methods Mol. Biol. 2018, 1721, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]




| SFN | FLZ | ||||
|---|---|---|---|---|---|
| Antimicrobial Activities | Pa | Pi | Antimicrobial Activities | Pa | Pi |
| Anti-Helicobacter pylori | 0.742 | 0.002 | Lanosterol 14 alpha demethylase inhibitor | 0.846 | 0.001 |
| Yeast RNA Inhibitor | 0.444 | 0.019 | Steroid synthesis inhibitor | 0.744 | 0.001 |
| Glycoprotein-phosphatidylinositol inhibitor | 0.516 | 0.093 | Antifungal | 0.726 | 0.008 |
| Antiparasitic | 0.441 | 0.023 | Phospholipid translation ATPase Inhibitor | 0.480 | 0.069 |
| Omptin inhibitor | 0.469 | 0.091 | NADPH-cytochrome-c2 reductase inhibitor | 0.366 | 0.134 |
| Phospholipid translation ATPase Inhibitor | 0.363 | 0.140 | Sugar-phosphatase inhibitor | 0.356 | 0.148 |
| Endopeptidase So inhibitor | 0.327 | 0.101 | Cell wall synthesis inhibitor | 0.351 | 0.002 |
| Mannose isomerase inhibitor | 0.302 | 0.071 | |||
| P. gingivalis TPR protease inhibitor | 0.301 | 0.108 | |||
| Estimated Oral Bioavailability | SFN | FLZ |
|---|---|---|
| iLogP | 2.11 | 0.41 |
| MW (g/mol) | 177.29 | 306.27 |
| TPSA | 29.43 Ų | 81.65 Ų |
| nHBD | 2 | 1 |
| nHBA | 0 | 7 |
| Predicted toxic effects | ||
| Mutagenic effects | Moderate | No |
| Tumorigenic effects | Moderate | No |
| Irritant effects | None | No |
| Hepatotoxicity | None | No |
| Effects on reproduction | Moderate | No |
| LD50 | 1000 mg/kg | 1410 mg/kg |
| Toxicity class | 4 | 4 |
| Estimated absorption | ||
| GI absorption | High | High |
| BBB permeability | No | No |
| Log Kp | −6.38 cm/s | −7.92 cm/s |
| Predicted solubility and drug-likeness and score | ||
| Log S | −2.10 | −2.17 |
| DL | −6.47 | 1.99 |
| DS | 0.25 | 0.87 |
| Strain | MIC (µg/mL) | MFC (µg/mL) | ||
|---|---|---|---|---|
| SFN | FLZ | SFN | FLZ | |
| ATCC 90028 | 30 | 1 | 60 | 8 |
| Oral 37 HIV+ | 60 | 16 | 240 | >64 |
| Oral 38 HIV+ | 30 | 4 | 30 | 64 |
| Oral 40 HIV+ | 30 | 8 | 60 | 64 |
| Strains | Mean FICI (μg/mL) | Interaction |
|---|---|---|
| C. albicans ATCC 90028 | 2.197 | Indifferent |
| C. albicans Oral 37 HIV+ | 1.412 | Indifferent |
| C. albicans Oral 40 HIV+ | 1.359 | Indifferent |
| Strain | FIC (μg/mL) at an SFN Concentration (μg/mL) of: | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| FLZ (μg/mL) | MIC/ 256 | MIC/ 128 | MIC/64 | MIC/ 32 | MIC/16 | MIC/8 | MIC/4 | MIC/2 | |
| ATCC 90028 | MIC/2 | 0.504 | 0.508 | 0.515 | 0.531 | 0.562 | 0.625 | 0.75 | 1 |
| Oral 37 HIV+ | MIC/4 | - | - | 0.265 | 0.281 | 0.312 | 0.375 | 0.5 | 0.75 |
| Oral 40 HIV+ | MIC/4 | 0.253 | 0.25 | 0.265 | 0.281 | 0.312 | 0.375 | 0.5 | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, B.L.R.; Simão, G.; Campos, C.D.L.; Monteiro, C.R.A.V.; Bueno, L.R.; Ortis, G.B.; Mendes, S.J.F.; Moreira, I.V.; Maria-Ferreira, D.; Sousa, E.M.; et al. In Silico and In Vitro Analysis of Sulforaphane Anti-Candida Activity. Antibiotics 2022, 11, 1842. https://doi.org/10.3390/antibiotics11121842
Silva BLR, Simão G, Campos CDL, Monteiro CRAV, Bueno LR, Ortis GB, Mendes SJF, Moreira IV, Maria-Ferreira D, Sousa EM, et al. In Silico and In Vitro Analysis of Sulforaphane Anti-Candida Activity. Antibiotics. 2022; 11(12):1842. https://doi.org/10.3390/antibiotics11121842
Chicago/Turabian StyleSilva, Bruna L. R., Gisele Simão, Carmem D. L. Campos, Cinara R. A. V. Monteiro, Laryssa R. Bueno, Gabriel B. Ortis, Saulo J. F. Mendes, Israel Viegas Moreira, Daniele Maria-Ferreira, Eduardo M. Sousa, and et al. 2022. "In Silico and In Vitro Analysis of Sulforaphane Anti-Candida Activity" Antibiotics 11, no. 12: 1842. https://doi.org/10.3390/antibiotics11121842
APA StyleSilva, B. L. R., Simão, G., Campos, C. D. L., Monteiro, C. R. A. V., Bueno, L. R., Ortis, G. B., Mendes, S. J. F., Moreira, I. V., Maria-Ferreira, D., Sousa, E. M., Vidal, F. C. B., Monteiro, C. d. A., Monteiro-Neto, V., & Fernandes, E. S. (2022). In Silico and In Vitro Analysis of Sulforaphane Anti-Candida Activity. Antibiotics, 11(12), 1842. https://doi.org/10.3390/antibiotics11121842