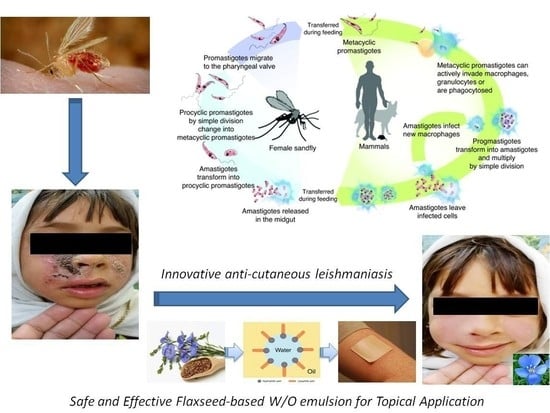
Fabrication and Evaluation of W/O Emulsion Loaded with Linum usitatissimum Seeds Extract for Anti-Leishmaniasis Efficacy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Screening: Qualitative and Quantitative Evaluations
2.2. Stability of Cream
2.3. Viscosity of Prepared LU Cream
2.4. In-Vitro Leishmanicidal Activity of LU against L. major Promastigotes
2.5. In-Vitro Leishmanicidal Activity of LU Seeds Extract against Intracellular Amastigotes
2.6. In-Vivo Anti-Leishmanial Efficacy of LU Seeds Extract in a Clinical Setting
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Plant Collection and Preparation of Crude Plant Extracts
3.3. Phytochemical Screening
3.3.1. Total Phenolic Content
3.3.2. Terpenoids
3.3.3. Flavonoids
3.3.4. Tannins
3.3.5. Saponins
3.3.6. Glycosides
3.3.7. Alkaloids
3.3.8. Reducing Sugar
3.4. Preparation of LU-Based Emulsion for Topical Application
3.5. Pharmaceutical Stability Test
3.6. Viscosity Measurement of Cream
3.7. In-Vitro Leishmanicidal Activity of LU against Promastigotes
3.8. In-Vitro Leishmanicidal Activity of LU Seeds Extract against Intracellular Amastigotes
3.9. In-Vivo Anti-Leishmanial Efficacy of LU Seeds Extract
3.10. Ethical Consideration
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000 Res. 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- De Vries, H.J.; Reedijk, S.H.; Schallig, H.D. Cutaneous leishmaniasis: Recent developments in diagnosis and management. Am. J. Clin. Dermatol. 2015, 16, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Munir, S.; Ayaz, S.; Khattak, B.U.; Khan, T.A.; Muhammad, N.; Anees, M.; Rahman, H.; Qasim, M.; Jamal, M.A.; et al. First report on molecular characterization of Leishmania species from cutaneous leishmaniasi spatients in southern Khyber Pakhtunkhwa Province of Pakistan. Asian Pac. J. Trop. Med. 2017, 10, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Savioli, L.; Velayudhan, R. Small bite, big threat: World Health Day 2014. East. Mediterr. Health J. Rev. St. Méditerranée Orient. 2014, 20, 217–218. [Google Scholar] [CrossRef]
- Hassan, A.H.E.; Phan, T.-N.; Yoon, S.; Lee, C.J.; Jeon, H.R.; Kim, S.; No, J.H.; Lee, Y.S. Pyrrolidine-based 3-deoxysphingosylphosphorylcholine analogs as possible candidates against neglected tropical diseases (NTDs): Identification of hit compounds towards development of potential treatment of Leishmania donovani. J. Enzym. Inhib. Med. Chem. 2021, 36, 1922–1930. [Google Scholar] [CrossRef]
- Hodiamont, C.J.; Kager, P.A.; Bart, A.; de Vries, H.J.; van Thiel, P.P.; Leenstra, T.; de Vries, P.J.; van Vugt, M.; Grobusch, M.P.; van Gool, T. Species-directedtherapy for leishmaniasisin returning travellers: Comprehensive guide. PLoS Negl. Trop. Dis. 2014, 8, e2832. [Google Scholar] [CrossRef]
- Alviano, D.S.; Barreto, A.L.S.; de Almeida Dias, F.; de Almeida Rodrigues, I.; dos Santos Rosa, M.D.S.; Alviano, C.S.; de Araújo Soares, R.M. Conventional therapy and promising plant-derived compounds against trypanosomatid parasites. Front. Microbiol. 2012, 3, 283. [Google Scholar] [CrossRef] [Green Version]
- Blum, J.; Neumayr, A.; Lockwood, D. Treatment of Tegumentary Forms of Leishmaniasis. In The Leishmaniases: Old Neglected Tropical Diseases; Springer: Berlin/Heidelberg, Germany, 2018; pp. 191–225. [Google Scholar]
- Chouhan, G.; Islamuddin, M.; Sahal, D.; Afrin, F. Exploring the role of medicinal plant- based immunomodulators for effective therapy of leishmaniasis. Front. Immunol. 2014, 5, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Adebayo, O.L.; Suleman, D.; Samson, A.A. Natural products in antileishmanial drug discovery: A review. J. Asian Sci. Res. 2013, 3, 157–161. [Google Scholar]
- Oryan, A. Plant-derived compounds in treatment of leishmaniasis. Iran. J. Vet. Res. 2015, 16, 1–8. [Google Scholar]
- Sifaoui, I.; López-Arencibia, A.; Martín-Navarro, C.M.; Chammem, N.; Reyes-Batlle, M.; Mejri, M.; Lorenzo-Morales, J.; Abderabba, M.; Piñero, J.E. Activity of olive leaf extracts against the promastigote stage of Leishmania species and their correlation with the antioxidant activity. Exp. Parasitol. 2014, 141, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Leon, R.; Vera-Ku, M.; Peraza-Sanchez, S.R.; Ku-Chulim, C.; Horta-Baas, A.; Rosado-Vallado, M. Antileishmanial activity of a mixture of Tridaxprocumbens and Allium sativum in mice. Parasite 2014, 21, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, R.; Haouas, N.; Ben Kahla-Nakbi, A.; Hammami, S.; Mighri, Z.; Mhenni, F.; Babba, H. The effect of vitis vinifera L. leaves extract on leishmania infantum. Iran. J. Pharm. Res. 2013, 12, 349–355. [Google Scholar] [PubMed]
- Rondon, F.C.; Bevilaqua, C.M.; Accioly, M.P.; de Morais, S.M.; de Andrade-Junior, H.F.; de Carvalho, C.A.; Lima, J.C.; Magalhães, H.C.R. In vitro efficacy of Coriandrum sativum, Lippia sidoides and Copaifera reticulata against Leishmania chagasi. Rev. Bras. Parasitol. Vet. 2012, 21, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Sachdeva, H.; Sehgal, R.; Kaur, S. Tinospora cordifolia as a protective and immunomodulatory agent in combination with cisplatin against murine visceral leishmaniasis. Exp. Parasitol. 2014, 137, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, H.; Sehgal, R.; Kaur, S. Asparagus racemosus ameliorates cisplatin induced toxicities and augments its antileishmanial activity by immunomodulation in vivo. Parasitol. Int. 2014, 63, 21–30. [Google Scholar] [CrossRef]
- Soosaraei, M.; Fakhar, M.; Hosseini Teshnizi, S.; Ziaei Hezarjaribi, H.; Banimostafavi, E.S. Medicinal plants with promising antileishmanial activity in Iran: A systematic revie and meta-analysis. Ann. Med. Surg. 2017, 21, 63–80. [Google Scholar] [CrossRef]
- Ogeto, T.K.; Odhiambo, R.A.; Shivairo, R.S.; Muleke, C.I.; Osero, B.O.; Anjili, C. Antileishmanial activity of Aloe secundiflora plant extracts against Leishmania major. Adv. Life Sci. Technol. 2013, 13, 9–18. [Google Scholar]
- Iqbal, H. Comparative efficacy of Aloe vera and Tamarixaphylla against cutaneous leishmaniasis. Int. J. Basic Med. Sci. Pharm. 2012, 2, 42–45. [Google Scholar]
- Abbasi, B.H.; Anjuma, S.; Hano, C. Differential effects of in vitro cultures of Linum usitatissimum L. (Flax) on biosynthesis, stability, antibacterial and antileishmanial activities of zinc oxide nanoparticles: A mechanistic approach. RSC Adv. 2017, 7, 15931. [Google Scholar] [CrossRef] [Green Version]
- Al-Sugmiany, R.Z.; Abed, A.M.; Al-Doori, M.A.M.; AL-Azzawie, A.F. Detection of the morphological and genetic effect of chloroformic flax seed extract on the Leishmania tropica. Drug Invent. Today 2020, 14, 897–903. [Google Scholar]
- Jabeen, N.; Maqbool, Q.; Sajjad, S.; Minhas, A.; Younas, U.; Anwaar, S.; Hussain, S.Z. Biosynthesis and characterisation of nano-silica as potential system for carrying streptomycin at nano-scale drug delivery. IET Nanobiotechnol. 2017, 11, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Barkat, A.; Naveed, A.; Tariq, M.; Qayum, M.; Shahiq, U.Z. Formulation and pharmaceutical evaluation of a W/O emulsion of Hippo phaerhamnoides. J. Pharm. Res. 2010, 6, 342–344. [Google Scholar]
- Sarah, J.M.; Maria, J. Phytochemical screening of flaxseed (Linum usitatissimum L.). Int. J. Sci. Res. 2016, 5, 218–220. [Google Scholar]
- Weniger, B.; Robledo, S.; Arango, G.J.; Deharo, E.; Aragón, R.; Muñoz, V.; Callapa, J.; Lobstein, A.; Anton, R. Antiprotozoal activities of Colombian plants. J. Ethnopharmacol. 2001, 78, 193–200. [Google Scholar] [CrossRef]
- Alamzeb, M.; Ali, S.; Khan, B.; Ihsanullah; Adnan; Omer, M.; Ullah, A.; Ali, J.; Setzer, W.N.; Salman, S.M.; et al. Antileishmanial Potential of Berberine Alkaloids from Berberis glaucocarpa Roots: Molecular Docking Suggests Relevant Leishmania Protein Targets. Nat. Prod. Commun. 2021, 16, 1934578X211031148. [Google Scholar]
- Sofowora, A. Medicinal Plants and Traditional Medicinal in Africa, 2nd ed.; Screening Plants for Bioactive Agents; Spectrum Books Ltd.: Ibadan, Nigeria, 1993; pp. 134–156. [Google Scholar]
- Trease, G.E.; Evans, W.C. Pharmacognosy, 15th ed.; Saunders Publishers: London, UK, 2002; pp. 42–44. [Google Scholar]
- Anwar, F.; Przybylski, R. Effect of solvents extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.). Acta Sci. Pol. Technol. Aliment. 2012, 11, 293–302. [Google Scholar]
- Akhtar, N.; Anwar, M.; Khan, B.A.; Mahmood, T.; Zaman, S.U. Formulation development and pharmaceutical evaluation of a W/O emulsion of Coleus extract. Indian J. Pharm. Educ. Res. 2011, 45, 236–241. [Google Scholar]
- Sharif, A.; Akhtar, N.; Khan, M.S.; Menaa, A.; Menaa, B.; Khan, B.A.; Menaa, F. Formulation and evaluation on human skin of a water-in-oil emulsion containing Muscat hamburg black grape seed extract. Int. J. Cosmet. Sci. 2015, 37, 253–258. [Google Scholar] [CrossRef]

| Phytochemicals | (Linum usitatissimum) |
|---|---|
| Phenols | + |
| Terpenoids | + |
| Flavonoids | + |
| Tannins | + |
| Saponins | − |
| Glycosides | − |
| Alkaloids | + |
| Carbohydrates | − |
| Time | Viscosities at 8 °C | Viscosities at 25 °C | Viscosities at 40 °C |
|---|---|---|---|
| Day 0 | 13,380 | 13,380 | 13,380 |
| Day 1 | 13,380 ± 13.8 | 13,370 ± 12.1 | 13,201 ± 10.2 |
| Day 2 | 13,378 ± 12.6 | 13,340 ± 12.3 | 13,100 ± 11.3 |
| Day 7 | 13,350 ± 13.3 | 13,310 ± 12.7 | 12,900 ± 11.3 |
| Day 14 | 13,342 ± 13.4 | 13,290 ± 11.6 | 12,870 ± 11.5 |
| Day 28 | 13,337 ± 12.2 | 13,270 ± 11.8 | 12,700 ± 13.2 |
| Test Samples | IC50 (µg/mL ± STD) | Maximum LU. Inhibition (%) | Amphotericin-B Inhibition (%) | Negative Control Inhibition (%) | Comments |
|---|---|---|---|---|---|
| Crude powder | 22.93 ± 0.2 | 67.85 | 100 | 0 | LAA |
| Crude extract | 19.51 ± 0.3 | 69.91 | 100 | 0 | IAA |
| Liquefied Cream * | 18.23 ± 1.1 | 73.85 | 100 | 0 | HAA |
| Number (#) of patients | 26 |
|---|---|
| Mean # of lesions per patient | 1.2 |
| Mean lesion size SD (mm) | 25.3 ± 0.1 |
| # of cured patients | 18 |
| # of patients for whom therapy failed | 8 |
| Cure rate in percentage (%) | 69.23% |
| Ingredients | Percent Used | |
|---|---|---|
| 01 | α Liquid Paraffin | 16 |
| 02 | α ABIL EM-90® | 5 |
| 03 | α Bees wax | 4 |
| 04 | β Plant Extract | 10 |
| 05 | β Fragrance | 1 |
| 06 | β D/W | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, B.A.; Faiz, S.; Khan, M.K.; Menaa, F.; Olah, N.-K.; Almoshari, Y.; Alamoudi, J.A.; Almawash, S. Fabrication and Evaluation of W/O Emulsion Loaded with Linum usitatissimum Seeds Extract for Anti-Leishmaniasis Efficacy. Antibiotics 2022, 11, 432. https://doi.org/10.3390/antibiotics11040432
Khan BA, Faiz S, Khan MK, Menaa F, Olah N-K, Almoshari Y, Alamoudi JA, Almawash S. Fabrication and Evaluation of W/O Emulsion Loaded with Linum usitatissimum Seeds Extract for Anti-Leishmaniasis Efficacy. Antibiotics. 2022; 11(4):432. https://doi.org/10.3390/antibiotics11040432
Chicago/Turabian StyleKhan, Barkat Ali, Sumera Faiz, Muhammad Khalid Khan, Farid Menaa, Neli-Kinga Olah, Yosif Almoshari, Jawaher Abdullah Alamoudi, and Saud Almawash. 2022. "Fabrication and Evaluation of W/O Emulsion Loaded with Linum usitatissimum Seeds Extract for Anti-Leishmaniasis Efficacy" Antibiotics 11, no. 4: 432. https://doi.org/10.3390/antibiotics11040432
APA StyleKhan, B. A., Faiz, S., Khan, M. K., Menaa, F., Olah, N. -K., Almoshari, Y., Alamoudi, J. A., & Almawash, S. (2022). Fabrication and Evaluation of W/O Emulsion Loaded with Linum usitatissimum Seeds Extract for Anti-Leishmaniasis Efficacy. Antibiotics, 11(4), 432. https://doi.org/10.3390/antibiotics11040432