Antiamoebic Properties of Laboratory and Clinically Used Drugs against Naegleria fowleri and Balamuthia mandrillaris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Henrietta Lacks (HeLa) and HaCaT Cell Culture
2.2. Culture of Amoebae
2.3. Chemicals
2.4. Amoebicidal Assays
2.5. Excystation Assays
2.6. Cytotoxicity Assays
3. Results and Discussion
3.1. Selected Compounds Exhibited Potent Amoebicidal Activities against N. fowleri
3.2. Selected Compounds Showed Anti-B. mandrillaris Properties
3.3. Excystation of N. fowleri and B. mandrillaris was Inhibited by Selected Compounds
3.4. All of the Compounds Tested Exhibited Minimal Cytotoxicity against Human Epithelial Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinez, A.J. Free-Living Amoebas; Natural History, Prevention, Diagnosis, Pathology and Treatment Of Disease; CRC Press Inc.: Boca Raton, FL, USA, 1985. [Google Scholar]
- Schuster, F.L.; Visvesvara, G.S. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 2004, 34, 1001–1027. [Google Scholar] [CrossRef] [PubMed]
- Visvesvara, G.S. Infections with free-living amebae. Handb. Clin. Neurol. 2013, 114, 153–168. [Google Scholar] [PubMed]
- Mungroo, M.R.; Khan, N.A.; Siddiqui, R. Naegleria fowleri: Diagnosis, treatment options and pathogenesis. Expert. Opin. Orphan. Drugs. 2019, 7, 67–80. [Google Scholar] [CrossRef]
- Heggie, T.W. Swimming with death: Naegleria fowleri infections in recreational waters. Travel. Med. Infect. Dis. 2010, 8, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Kalsoom, H.; Baig, A.M.; Khan, N.A.; Siddiqui, R. Laboratory testing of clinically approved drugs against Balamuthia mandrillaris. World J. Microbiol. Biotechnol. 2014, 30, 2337–2342. [Google Scholar] [CrossRef]
- Laurie, M.T.; White, C.V.; Retallack, H.; Wu, W.; Moser, M.S.; Sakanari, J.A.; Ang, K.; Wilson, C.; Arkin, M.R.; DeRisi, J.L. Functional Assessment of 2,177 US and International Drugs Identifies the Quinoline Nitroxoline as a Potent Amoebicidal Agent against the Pathogen Balamuthia mandrillaris. mBio 2018, 9, e02051–e02118. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, R.; Khan, N.A. Balamuthia amoebic encephalitis: An emerging disease with fatal consequences. Microb. Pathog. 2008, 44, 89–97. [Google Scholar] [CrossRef]
- Maciver, S.K.; Piñero, J.E.; Lorenzo-Morales, J. Is Naegleria fowleri an emerging parasite? Trends Parasitol. 2020, 36, 19–28. [Google Scholar] [CrossRef]
- Siddiqui, R.; Ali, I.K.M.; Cope, J.R.; Khan, N.A. Brain-Eating Amoebae; Caister Academic Press: Poole, UK, 2016; pp. 21–26. [Google Scholar]
- Anwar, A.; Khan, N.A.; Siddiqui, R. Repurposing of Drugs Is a Viable Approach to Develop Therapeutic Strategies against Central Nervous System Related Pathogenic Amoebae. ACS Chem. Neurosci. 2020, 11, 2378–2384. [Google Scholar] [CrossRef]
- Anwar, A.; Mungroo, M.R.; Anwar, A.; Sullivan, W.J., Jr.; Khan, N.A.; Siddiqui, R. Repositioning of guanabenz in conjugation with gold and silver nanoparticles against pathogenic amoebae Acanthamoeba castellanii and Naegleria Fowleri. ACS Infect. Dis. 2019, 5, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.A.; Colon, B.L.; Chen, E.; Hull, M.V.; Kyle, D.E. Discovery of repurposing drug candidates for the treatment of diseases caused by pathogenic free-living amoebae. PLoS Negl. Trop. Dis. 2020, 14, e0008353. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Soomaroo, A.; Anwar, A.; Siddiqui, R.; Khan, N.A. Metformin-coated silver nanoparticles exhibit anti-acanthamoebic activities against both trophozoite and cyst stages. Exp. Parasitol. 2020, 215, 107915. [Google Scholar] [CrossRef]
- García-Angulo, P.; Alonso-Simón, A.; Encina, A.; Álvarez, J.M.; Acebes, J.L. Cellulose biosynthesis inhibitors: Comparative effect on bean cell cultures. Int. J. Mol. Sci. 2012, 13, 3685–3702. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, D.J.; Fleming, M.B.; Patterson, E.L.; Sebastian, J.R.; Nissen, S.J. Indaziflam: A new cellulose-biosynthesis-inhibiting herbicide provides long-term control of invasive winter annual grasses. Pest Manag. Sci. 2017, 73, 2149–2162. [Google Scholar] [CrossRef] [Green Version]
- Scheible, W.R.; Fry, B.; Kochevenko, A.; Schindelasch, D.; Zimmerli, L.; Somerville, S.; Loria, R.; Somerville, C.R. An Arabidopsis mutant resistant to thaxtomin A, a cellulose synthesis inhibitor from Streptomyces species. Plant Cell 2003, 15, 1781–1794. [Google Scholar] [CrossRef] [Green Version]
- Sahid, I.; Razlin, W.; Zaabar, W. Effects of thiram and terbuthylazine on cellulose decomposition in two soils. Bull. Environ. Contam. Toxicol. 1993, 51, 605–611. [Google Scholar] [CrossRef]
- Dunn, C.J.; Faulds, D. Nateglinide. Drugs 2000, 60, 607–615. [Google Scholar] [CrossRef]
- Siddiqui, R.; Jarroll, E.L.; Khan, N.A. Balamuthia mandrillaris: Staining Properties of Cysts and Trophozoites and the Effect of 2, 6-Dichlorobenzonitrile and Calcofluor White on Encystment. J. Eukaryot. Microbiol. 2009, 56, 136–141. [Google Scholar] [CrossRef]
- Rajendran, K.; Anwar, A.; Khan, N.A.; Shah, M.R.; Siddiqui, R. Trans-Cinnamic acid conjugated gold nanoparticles as potent therapeutics against brain-eating amoeba Naegleria Fowleri. ACS Chem. Neurosci. 2019, 10, 2692–2696. [Google Scholar] [CrossRef]
- Jeyamogan, S.; Khan, N.A.; Anwar, A.; Shah, M.R.; Siddiqui, R. Cytotoxic effects of Benzodioxane, Naphthalene diimide, Porphyrin and Acetamol derivatives on HeLa cells. SAGE Open Med. 2018, 6, 2050312118781962. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, K.; Anwar, A.; Khan, N.A.; Siddiqui, R. Brain-eating amoebae: Silver nanoparticle conjugation enhanced efficacy of anti-amoebic drugs against Naegleria fowleri. ACS Chem. Neurosci. 2017, 8, 2626–2630. [Google Scholar] [CrossRef]
- Mungroo, M.R.; Anwar, A.; Khan, N.A.; Siddiqui, R. Gold-Conjugated Curcumin as a Novel Therapeutic Agent against Brain-Eating Amoebae. ACS Omega 5 2020, 5, 12467–12475. [Google Scholar] [CrossRef]
- Drugbank. Available online: https://www.drugbank.ca/drugs/ (accessed on 2 January 2021).
- Liu, T.B.; Kim, J.C.; Wang, Y.; Toffaletti, D.L.; Eugenin, E.; Perfect, J.R.; Kim, K.J.; Xue, C. Brain inositol is a novel stimulator for promoting Cryptococcus penetration of the blood-brain barrier. PLoS Pathog. 2013, 9, e1003247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Center for Biotechnology Information (PubChem Compound Summary). Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 2 January 2021).
- Masri, A.; Anwar, A.; Ahmed, D.; Siddiqui, R.B.; Raza Shah, M.; Khan, N.A. Silver nanoparticle conjugation-enhanced antibacterial efficacy of clinically approved drugs cephradine and vildagliptin. Antibiotics 2018, 7, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debnath, A. Drug discovery for primary amebic meningoencephalitis: From screen to identification of leads. Expert Rev. Anti-infect. Ther. 2021, 19, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Khan, N.A. Proposed intranasal route for drug administration in the management of central nervous system manifestations of COVID-19. ACS Chem. Neurosci. 2020, 11, 1523–1524. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Abouleish, M.Y.; Khamis, M.; Ibrahim, T.; Khan, N.A. Potential Application of Vaporized Drugs via Nasal Inhalers to Prevent Mortality and Central Nervous System Damage Caused by Primary Amoebic Meningoencephalitis Due to Naegleria fowleri. ACS Pharmacol. Transl. Sci. 2021, 4, 1249–1252. [Google Scholar] [CrossRef]
- Khafagy, R.; Gupta, S.; Campisi, P.; Waters, V. Treatment of localized mucormycosis using nasal amphotericin B irrigation in pediatric oncology. Pediatr. Blood Cancer 2020, 67, e28175. [Google Scholar] [CrossRef]




| Compound | Trade Name | Emperical Formula | IUPAC Name | Molar Mass | Mechanism of Action | BBB Permeability | Solvent |
|---|---|---|---|---|---|---|---|
| Metformin | Glucophage, Riomet, Fortamet, etc. | C4H11N5 | 1-carbamimidamido-N,N-dimethylmethanimidamide | 165.63 | Protein kinase activity inducer; Ubiquinone binding inhibitor; NAD binding inhibitor | Positive | RPMI-1640 |
| Quinclorac | Not used clinically | C10H5Cl2NO2 | 3,7-Dichloro-8-quinolinecarboxylic acid | 242.06 | Acetylcholinesterase inihbitor; binds to albumin | Not available | RPMI-1640 |
| Indaziflam | Not used clinically | C16H20FN5 | N-(2,3-dihydro-2,6-dimethyl-1H-inden-1-yl)-6-(1-fluoroethyl)-1,3,5-triazine-2,4-diamine | 301.36 | Cellulose biosynthesis inhibitor | Not available | RPMI-1640 |
| Inositol | Ovasitol, Inositech, Niacinol, etc. | C6H12O6 | cyclohexane-1,2,3,4,5,6-hexol | 180.16 | Phosphoric diester hydrolase activity; Glucosylceramidase receptor binding; Manganese ion binding inhibitor | Present in brain | RPMI-1640 |
| Nateglinide | Starlix | C19H27NO3 | (2R)-3-phenyl-2-[(4-propan-2-ylcyclohexanecarbonyl)amino]propanoic acid | 317.43 | Sulfonylurea receptor inhibitor; Zinc ion binding agonist | Positive | 20% Ethanol |
| Dichlobenil (2,6-DNBT) | Not used clinically | C7H3Cl2N | 2,6-dichlorobenzonitrile | 172.01 | Cellulose synthesis inhibitor | Not available | 20% Ethanol |
| Trans-cinnamic acid | Not used clinically | C9H8O2 | (E)-3-phenylprop-2-enoic acid | 148.16 | Hydroxycarboxylic acid receptors agonist | Not available | 20% Ethanol |
| Terbuthylazine | Not used clinically | C9H16ClN5 | 2-N-tert-butyl-6-chloro-4-N-ethyl-1,3,5-triazine-2,4-diamine | 229.71 | Triazine selective systemic herbicide affecting electron transport | Not available | 20% DMSO |
| Acarbose | Precose | C25H43NO18 | (3R,4R,5S,6R)-5-[(2R,3R,4R,5S,6R)-5-[(2R,3R,4S,5S,6R)-3,4-dihydroxy-6-methyl-5-[[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-en-1-yl]amino]oxan-2-yl]oxy-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-6-(hydroxymethyl)oxane-2,3,4-triol | 645.61 | alpha-glucosidases inhibitor | Negative | 20% DMSO |
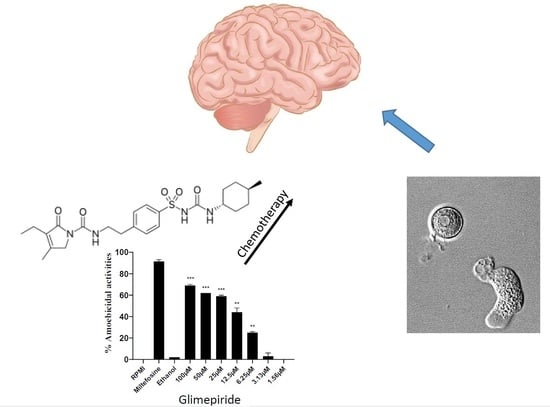
| Glimepiride | Amaryl | C24H34N4O5S | 4-ethyl-3-methyl-N-[2-[4-[(4-methylcyclohexyl)carbamoylsulfamoyl]phenyl]ethyl]-5-oxo-2H-pyrrole-1-carboxamide | 490.62 | Voltage-gated potassium channel inhibitor; Phosphatidylinositol-4,5-bisphosphate binding inhibitor; Sulfonylurea receptor inducer | Positive | 20% DMSO |
| Vildagliptin | Galvus | C17H25N3O2 | (2S)-1-[2-[(3-hydroxy-1-adamantyl)amino]acetyl]pyrrolidine-2-carbonitrile | 303.4 | Dipeptidyl peptidase 4 inhibitor | Positive | RPMI-1640 |
| Cellulase | Not used clinically | C18H32O16 | (2S,3R,4S,5S,6R)-2-[(2R,3S,4R,5R,6S)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(2R,3S,4R,5R,6R)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | 504.4 | Hydrolysis of 1,4-beta-glucosidic linkages in cellulose | Not available | RPMI-1640 |
| Thaxtomin A | Supplied by Adipogen as antibiotic | C22H22N4O6 | (3R,6S)-3-hydroxy-3-[(3-hydroxyphenyl)methyl]-1,4-dimethyl-6-[(4-nitro-1H-indol-3-yl)methyl]piperazine-2,5-dione | 483.43 | Not Available | Not available | 20% DMSO |
| Repaglinide | Prandin, NovoNorm, Enyglid, etc. | C27H36N2O4 | 2-ethoxy-4-[2-[[(1S)-3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino]-2-oxoethyl]benzoic acid | 452.59 | Potassium voltage-gated channel inhibitor; Sulfonylurea receptor activity inhibitor; Zinc ion binding agonist | Negative | 20% DMSO |
| dimethyl peptidase (IV) inhibitor | Januvia, Onglyza, Tradjenta, etc. | Not Available | Not Available | 328.41 | Dipeptidyl peptidase 4 inhibitor | Negative | 20% DMSO |
| Compound | EC50 (μM) | |
|---|---|---|
| N. fowleri | B. mandrillaris | |
| Metformin | 134.0 | Not available |
| Quinclorac | 102.1 | 296.6 |
| Indaziflam | 53.7 | 99.8 |
| Inositol | 48.9 | 124.1 |
| Nateglinide | 44.4 | 136.2 |
| Dichlobenil (2,6-DNBT) | 24.2 | 39.6 |
| Trans-cinnamic acid | 9.4 | Not available |
| Terbuthylazine | 7.5 | 62.5 |
| Acarbose | 3.6 | 80.8 |
| Glimepiride | 5.2 | 63.6 |
| Vildagliptin | 100.3 | Not available |
| Cellulase | Not available | 106.0 |
| Thaxtomin A | 94.7 | 39.0 |
| Repaglinide | 14.3 | 31.8 |
| dimethyl peptidase (IV) inhibitor | 14.9 | 13.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, R.; Mungroo, M.R.; Anuar, T.S.; Alharbi, A.M.; Alfahemi, H.; Elmoselhi, A.B.; Khan, N.A. Antiamoebic Properties of Laboratory and Clinically Used Drugs against Naegleria fowleri and Balamuthia mandrillaris. Antibiotics 2022, 11, 749. https://doi.org/10.3390/antibiotics11060749
Siddiqui R, Mungroo MR, Anuar TS, Alharbi AM, Alfahemi H, Elmoselhi AB, Khan NA. Antiamoebic Properties of Laboratory and Clinically Used Drugs against Naegleria fowleri and Balamuthia mandrillaris. Antibiotics. 2022; 11(6):749. https://doi.org/10.3390/antibiotics11060749
Chicago/Turabian StyleSiddiqui, Ruqaiyyah, Mohammad Ridwane Mungroo, Tengku Shahrul Anuar, Ahmad M. Alharbi, Hasan Alfahemi, Adel B. Elmoselhi, and Naveed Ahmed Khan. 2022. "Antiamoebic Properties of Laboratory and Clinically Used Drugs against Naegleria fowleri and Balamuthia mandrillaris" Antibiotics 11, no. 6: 749. https://doi.org/10.3390/antibiotics11060749
APA StyleSiddiqui, R., Mungroo, M. R., Anuar, T. S., Alharbi, A. M., Alfahemi, H., Elmoselhi, A. B., & Khan, N. A. (2022). Antiamoebic Properties of Laboratory and Clinically Used Drugs against Naegleria fowleri and Balamuthia mandrillaris. Antibiotics, 11(6), 749. https://doi.org/10.3390/antibiotics11060749